CAR-redirected natural killer T cells demonstrate superior antitumor activity to CAR-T cells through multimodal CD1d-dependent mechanisms
IF 23.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
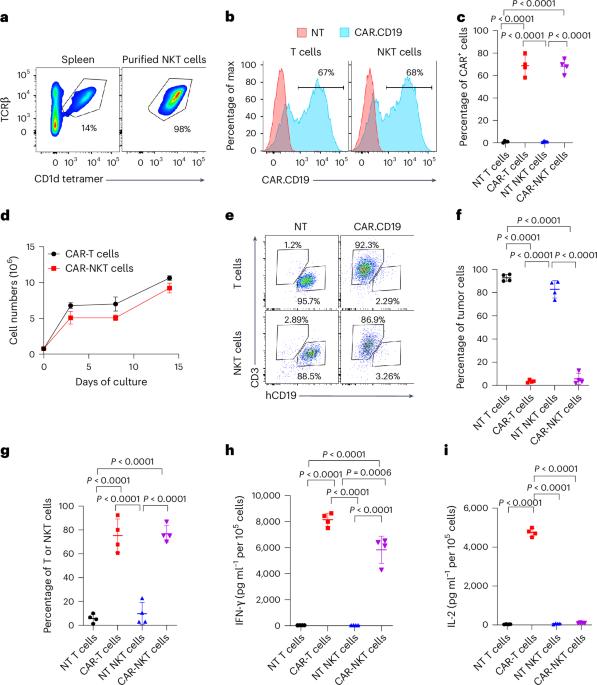
Human natural killer T (NKT) cells have been proposed as a promising cell platform for chimeric antigen receptor (CAR) therapy in solid tumors. Here we generated murine CAR-NKT cells and compared them with CAR-T cells in immune-competent mice. Both CAR-NKT cells and CAR-T cells showed similar antitumor effects in vitro, but CAR-NKT cells showed superior antitumor activity in vivo via CD1d-dependent immune responses in the tumor microenvironment. Specifically, we show that CAR-NKT cells eliminate CD1d-expressing M2-like macrophages. In addition, CAR-NKT cells promote epitope spreading and activation of endogenous T cell responses against tumor-associated neoantigens. Finally, we observed that CAR-NKT cells can co-express PD1 and TIM3 and show an exhaustion phenotype in a model of high tumor burden. PD1 blockade as well as vaccination augmented the antitumor activity of CAR-NKT cells. In summary, our results demonstrate the multimodal function of CAR-NKT cells in solid tumors, further supporting the rationale for developing CAR-NKT therapies in the clinic. Dotti and colleagues show that chimeric antigen receptor (CAR) natural killer T cells have superior antitumor activity compared with CAR-T cells, mediated through the elimination of CD1d-expressing tumor-associated macrophages, activation of dendritic cells and promotion of endogenous T cell responses.
CAR-redirected 自然杀伤 T 细胞通过 CD1d 依赖性多模式机制显示出优于 CAR-T 细胞的抗肿瘤活性。
人类自然杀伤 T(NKT)细胞被认为是一种有希望用于实体瘤嵌合抗原受体(CAR)疗法的细胞平台。在这里,我们生成了小鼠 CAR-NKT 细胞,并在免疫功能正常的小鼠体内将其与 CAR-T 细胞进行了比较。CAR-NKT 细胞和 CAR-T 细胞在体外表现出相似的抗肿瘤效果,但 CAR-NKT 细胞通过肿瘤微环境中 CD1d 依赖性免疫反应在体内表现出更优越的抗肿瘤活性。具体来说,我们发现 CAR-NKT 细胞能消灭表达 CD1d 的 M2 样巨噬细胞。此外,CAR-NKT 细胞还能促进表位扩散,激活内源性 T 细胞对肿瘤相关新抗原的反应。最后,我们观察到 CAR-NKT 细胞能同时表达 PD1 和 TIM3,并在高肿瘤负荷模型中表现出衰竭表型。PD1 阻断和疫苗接种增强了 CAR-NKT 细胞的抗肿瘤活性。总之,我们的研究结果证明了CAR-NKT细胞在实体瘤中的多模式功能,进一步支持了在临床中开发CAR-NKT疗法的理由。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature cancer
Medicine-Oncology
CiteScore
31.10
自引率
1.80%
发文量
129
期刊介绍:
Cancer is a devastating disease responsible for millions of deaths worldwide. However, many of these deaths could be prevented with improved prevention and treatment strategies. To achieve this, it is crucial to focus on accurate diagnosis, effective treatment methods, and understanding the socioeconomic factors that influence cancer rates.
Nature Cancer aims to serve as a unique platform for sharing the latest advancements in cancer research across various scientific fields, encompassing life sciences, physical sciences, applied sciences, and social sciences. The journal is particularly interested in fundamental research that enhances our understanding of tumor development and progression, as well as research that translates this knowledge into clinical applications through innovative diagnostic and therapeutic approaches. Additionally, Nature Cancer welcomes clinical studies that inform cancer diagnosis, treatment, and prevention, along with contributions exploring the societal impact of cancer on a global scale.
In addition to publishing original research, Nature Cancer will feature Comments, Reviews, News & Views, Features, and Correspondence that hold significant value for the diverse field of cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


