Genetic Analysis of Neurite Outgrowth Inhibitor-Associated Genes in Parkinson's Disease: A Cross-Sectional Cohort Study
Abstract
Background
Parkinson's disease (PD) is a neurodegenerative disease caused by a combination of aging, environmental, and genetic factors. Previous research has implicated both causative and susceptibility genes in PD development. Nogo-A, a neurite outgrowth inhibitor, has been shown to impact axon growth through ligand-receptor interactions negatively, thereby involved in the deterioration of dopaminergic neurons. However, rare genetic studies have identified the relationship between neurite outgrowth inhibitor (Nogo)-associated genes and PD from a signaling pathway perspective.
Methods
We enrolled 3959 PD patients and 2931 healthy controls, categorized into two cohorts based on their family history and age at onset: sporadic early Parkinson's disease & familial Parkinson's disease (sEOPD & FPD) cohort and sporadic late Parkinson's disease (sLOPD) cohort. We selected 17 Nogo-associated genes and stratified them into three groups via their function, respectively, ligand, receptors, and signaling pathway groups. Additionally, we conducted the burden analysis in rare variants, the logistic regression analysis in common variants, and the genotype–phenotype association analysis. Last, bioinformatics analysis and functional experiments were conducted to identify the role of the MTOR gene in PD.
Results
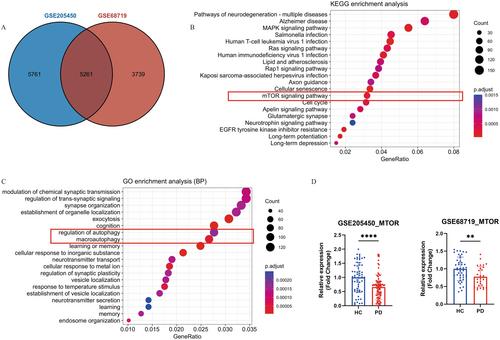
Our findings demonstrated that the missense variants in the MTOR gene might increase PD risk, while the deleterious variants in the receptor subtype of Nogo-associated genes might mitigate PD risk. However, common variants of Nogo-associated genes showed no association with PD development in two cohorts. Furthermore, genotype–phenotype association analysis suggested that PD patients with MTOR gene variants exhibited relatively milder motor symptoms but were more susceptible developing dyskinesia. Additionally, bioinformatics analysis results showed MTOR gene was significantly decreased in PD, indicating a potential negative role of the mTOR in PD pathogenesis. Experimental data further demonstrated that MHY1485, a mTOR agonist, could rescue MPP+-induced axon inhibition, further implicating the involvement of mTOR protein in PD by regulating cell growth and axon growth.
Conclusions
Our preliminary investigation highlights the association of Nogo-associated genes with PD onset in the Chinese mainland population and hints at the potential role of the MTOR gene in PD. Further research is warranted to elucidate the mechanistic pathways underlying these associations and their therapeutic implications.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


