Investigating how H2S can alter the interactions between Hg0 and corroded steel surfaces to guide future decommissioning projects
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Many oil and gas developments will soon be decommissioned and, knowledge on the accumulation of mercury (Hg), throughout offshore infrastructure is limited. Any release of Hg could have a detrimental impact on marine ecosystems. To bridge this knowledge gap, a fractionation approach was taken on steel samples exposed to Hg0 and H2S, separating Hg compounds removed from the surface into polar, non-polar and insoluble fractions. Hg0 reacted on corroded surfaces to form several compounds, over 50% of which were removed by seawater. This suggests that pipelines on the seabed could release a dramatic amount of Hg into the sea if they are left in place. Furthermore, a Cu-Hg amalgam, was identified to be a dominant species, by a combination of XFM, XANES and LA-ICP-TOFMS. Seawater-soluble and amalgam-bound Hg were present regardless of co-exposure to H2S. When H2S was present Hg nanoparticles accounted for up to 1% of the total Hg on the steel. This investigation has shown that the Hg speciation on the surfaces of pipelines is complex and future decommissioning strategies should consider a range of Hg species beyond only Hg0 and metacinnabar (β-HgS), all of which could interact with biota and impact Hg biomagnification through the marine the food web.
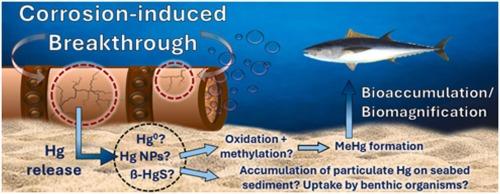
研究 H2S 如何改变 Hg0 与腐蚀钢表面之间的相互作用,以指导未来的退役项目
许多石油和天然气开发项目将很快退役,但人们对整个近海基础设施的汞(Hg)积累情况了解有限。汞的任何释放都会对海洋生态系统造成有害影响。为了弥补这一知识空白,我们对暴露于 Hg0 和 H2S 的钢材样本采用了分馏方法,将从表面去除的汞化合物分成极性、非极性和不溶性部分。Hg0 在腐蚀表面发生反应,形成多种化合物,其中 50% 以上被海水去除。这表明,如果海底管道留在原地,可能会向海洋释放大量的汞。此外,通过 XFM、XANES 和 LA-ICP-TOFMS 三种方法的综合应用,还确定了一种铜汞合金是主要物种。无论是否同时暴露于 H2S,海水可溶性汞和汞合金结合汞都存在。当 H2S 存在时,钢材上的汞纳米颗粒最多可占汞总量的 1%。这项调查表明,管道表面的汞标示非常复杂,未来的退役战略应考虑一系列汞物种,而不仅仅是 Hg0 和偏辰砂(β-HgS),所有这些都可能与生物群相互作用,并通过海洋食物网影响汞的生物放大作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


