Convergent synthesis and protein binding of vicinal difluorides by stereodivergent C–C bond formation
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Vicinal difluorides adopt defined conformations due to the electronic properties of fluorine. Therefore, they could be valuable for controlling the constellation of functional groups about acyclic C–C bonds in organic molecules if all stereoisomers of the difluorides could be synthesized. However, stereoselective synthesis of vicinal difluorides has been cumbersome. The location of functional groups within organic molecules is important because it influences function, particularly biological function. We report a catalytic synthesis of acyclic vicinal difluoride stereoisomers by C–C bond formation between two monofluoro units, along with crystallographic and computational data showing that the gauche relationship of two fluorides causes substituents to occupy defined positions about the C(sp3)–C(sp3) bond. Photoreactive chemical probes tethered to vicinal difluorides showed that difluorides bind more strongly than the analogous monofluorides, which possess less defined conformations, and that individual stereoisomers of the difluorides bind distinctly to the human proteome.
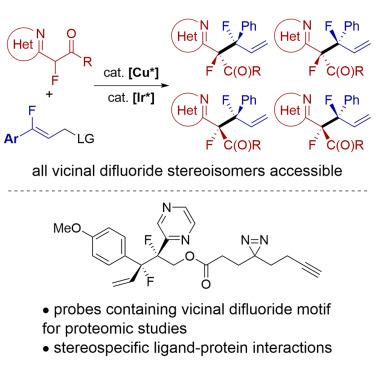

通过立体异构 C-C 键形成的邻位二氟化物的趋同合成和蛋白质结合
由于氟的电子特性,二氟化物具有明确的构象。因此,如果能合成二氟化物的所有立体异构体,它们在控制有机分子中无环 C-C 键官能团的排列方面就能发挥重要作用。然而,邻位二氟化物的立体选择性合成非常麻烦。有机分子中官能团的位置非常重要,因为它会影响功能,尤其是生物功能。我们报告了通过两个单氟单元之间的 C-C 键形成催化合成无环侧二氟化物立体异构体的方法,以及晶体学和计算数据,这些数据表明两个氟化物的高程关系会导致取代基占据关于 C(sp3)-C(sp3) 键的确定位置。与邻位二氟化物相连的光活性化学探针表明,二氟化物比具有较不确定构象的类似单氟化物的结合力更强,而且二氟化物的单个立体异构体与人类蛋白质组的结合也很明显。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


