Exploring the efficiency of caprylic acid and quaternary ammonium salt-based deep eutectic solvents for separation of CO2 and H2S from gas mixtures using molecular dynamics simulations and COSMO-RS
IF 5.5
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
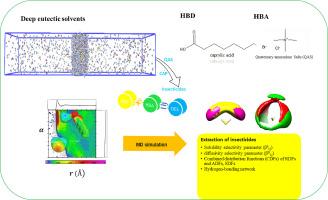
In the present study, quaternary ammonium salts (QAS) and caprylic acid based deep eutectic solvents (DES) were employed for the separation of CO2 and H2S from natural gas (NG). To elucidate the extraction mechanisms of H2S from natural gas (NG), we employed molecular dynamics (MD) simulations to investigate the intermolecular interactions between the gases and the eutectic solvents. Our comprehensive analysis aimed at enhancing the understanding of quaternary ammonium salt (QAS) absorption capability in H2S extractions including combined distribution functions (CDFs), which encompass radial distribution functions (RDFs), angular distribution functions (ADFs), hydrogen-bonding networks, density profiles, spatial distribution functions (SDFs), and the non-bond energies between H2S and the DES components, all measured at 310 K. Additionally, we calculated the solubility of H2S and CO2 gases as well as the solubility selectivity and diffusivity selectivity parameters to evaluate the performance of the QAS-based DES in sweetening processes of NG. The results represent that eutectic solvents based on quaternary ammonium chloride are effective for H2S adsorption and show selectivity for H2S in gas mixtures. Furthermore, the COSMO-RS model was used to predict the solubility of various gases (H2S/CO2) in the QAS-based DES, providing further insights into their effectiveness.
利用分子动力学模拟和 COSMO-RS 探索辛酸和季铵盐基深共晶溶剂从气体混合物中分离 CO2 和 H2S 的效率
本研究采用季铵盐(QAS)和辛酸基深共晶溶剂(DES)分离天然气(NG)中的 CO2 和 H2S。为了阐明天然气(NG)中 H2S 的萃取机理,我们采用分子动力学(MD)模拟来研究气体与共晶溶剂之间的分子间相互作用。我们的综合分析旨在加深对季铵盐(QAS)在 H2S 萃取过程中吸收能力的理解,包括组合分布函数(CDF),其中包括径向分布函数(RDF)、角分布函数(ADF)、氢键网络、密度曲线、空间分布函数(SDF)以及 H2S 与 DES 成分之间的非键能,所有这些都是在 310 K 条件下测量的。此外,我们还计算了 H2S 和 CO2 气体的溶解度以及溶解度选择性和扩散选择性参数,以评估基于 QAS 的 DES 在 NG 增甜过程中的性能。结果表明,基于季铵氯化物的共晶溶剂能有效吸附 H2S,并显示出对混合气体中 H2S 的选择性。此外,还利用 COSMO-RS 模型预测了各种气体(H2S/CO2)在基于季铵盐的 DES 中的溶解度,从而进一步了解了其有效性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal Advances
Engineering-Industrial and Manufacturing Engineering
CiteScore
8.30
自引率
0.00%
发文量
213
审稿时长
26 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


