Efficient transformation of CO2 into α-alkylidene cyclic carbonates over Cu0/Cu+ derived from CuAl layered double hydroxide/reduced graphene oxide hybrid
IF 5.3
2区 地球科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
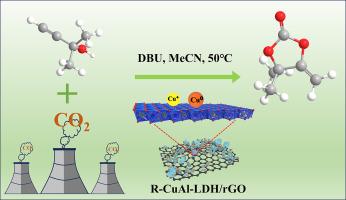
A simple liquid-phase co-precipitation method was used to achieve a good dispersion of CuAl layered double hydroxide (CuAl-LDH) on the surface of graphene oxide (GO), and Cu2+ in the lattice of CuAl-LDH was in-situ reduced to Cu0 and Cu+ species, resulting in the preparation of R-CuAl-LDH/rGO catalyst. It could effectively catalyze the cyclization of CO2 and propargylic alcohols under mild conditions (50 °C and 1 bar), and the yield exceeded 99 % within only 40 min. Systematic characterization revealed that two-dimensional LDH nanosheets were grown on the rGO surface in an irregular orientation, leading to a higher specific surface area and more abundant surface active sites. Meanwhile, the synergistic catalysis of Cu0 and Cu+ dual-active sites greatly enhanced the catalytic efficiency. In addition, the catalyst had excellent recycling properties with no significant loss of activity after 5 cycles of continuous operation.
在源自 CuAl 层状双氢氧化物/还原氧化石墨烯混合物的 Cu0/Cu+ 上将 CO2 高效转化为 α-亚烷基环碳酸盐
采用简单的液相共沉淀方法在氧化石墨烯(GO)表面实现了CuAl层状双氢氧化物(CuAl-LDH)的良好分散,并将CuAl-LDH晶格中的Cu2+原位还原为Cu0和Cu+物种,从而制备了R-CuAl-LDH/rGO催化剂。它能在温和的条件下(50 °C 和 1 bar)有效催化 CO2 和丙炔醇的环化反应,仅在 40 分钟内产率就超过了 99%。系统表征结果表明,二维 LDH 纳米片以不规则取向生长在 rGO 表面,从而获得了更高的比表面积和更丰富的表面活性位点。同时,Cu0 和 Cu+ 双活性位点的协同催化作用大大提高了催化效率。此外,该催化剂还具有优异的回收性能,在连续运行 5 个周期后活性没有明显降低。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Clay Science
地学-矿物学
CiteScore
10.30
自引率
10.70%
发文量
289
审稿时长
39 days
期刊介绍:
Applied Clay Science aims to be an international journal attracting high quality scientific papers on clays and clay minerals, including research papers, reviews, and technical notes. The journal covers typical subjects of Fundamental and Applied Clay Science such as:
• Synthesis and purification
• Structural, crystallographic and mineralogical properties of clays and clay minerals
• Thermal properties of clays and clay minerals
• Physico-chemical properties including i) surface and interface properties; ii) thermodynamic properties; iii) mechanical properties
• Interaction with water, with polar and apolar molecules
• Colloidal properties and rheology
• Adsorption, Intercalation, Ionic exchange
• Genesis and deposits of clay minerals
• Geology and geochemistry of clays
• Modification of clays and clay minerals properties by thermal and physical treatments
• Modification by chemical treatments with organic and inorganic molecules(organoclays, pillared clays)
• Modification by biological microorganisms. etc...
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


