Polydopamine-functionalized calcium-deficient hydroxyapatite 3D-printed scaffold with sustained doxorubicin release for synergistic chemo-photothermal therapy of osteosarcoma and accelerated bone regeneration
IF 8.7
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Interior bone-tissue regeneration and rapid tumor recurrence post-resection are critical challenges in osteosarcoma and other bone cancers. Conventional bone tissue engineering scaffolds lack inhibitory effects on bone tumor recurrence. Herein, multifunctional scaffolds (named DOX/PDA@CDHA) were designed through the spontaneous polymerization of Dopamine (PDA) on the surface of Calcium Deficient Hydroxyapatite (CDHA) scaffolds, followed by in situ loading of the chemotherapeutic drug Doxorubicin (DOX). The PDA coating endowed the scaffolds with significant photothermal properties, while the gradual release of DOX provided an effective chemotherapeutic effect. The on-demand release of DOX at tumor sites, triggered by dual stimulation (near-infrared (NIR) light and the acidic pH typical of tumor microenvironments), specifically targets cancer cells, thereby mitigating systemic side effects. These unique characteristics facilitated effective osteosarcoma eradication both in vitro and in vivo. Moreover, the scaffold's composition, which mimics the mineral phase of natural bone and is enhanced by PDA's biocompatibility, promotes critical osteogenic and angiogenic processes. This facilitates not only tumor eradication but also the regeneration of healthy bone tissue. Collectively, this study presents a potent candidate for the regeneration of bone defects induced by osteosarcoma.
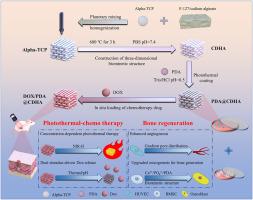
具有持续释放多柔比星功能的多巴胺功能化缺钙羟基磷灰石三维打印支架,用于骨肉瘤的协同化疗-光热疗法和加速骨再生
骨组织内部再生和肿瘤切除后的快速复发是骨肉瘤和其他骨癌面临的严峻挑战。传统的骨组织工程支架缺乏对骨肿瘤复发的抑制作用。本文通过在缺钙羟基磷灰石(CDHA)支架表面自发聚合多巴胺(PDA),然后原位负载化疗药物多柔比星(DOX),设计出多功能支架(命名为 DOX/PDA@CDHA)。PDA 涂层赋予了支架显著的光热特性,而 DOX 的逐步释放则提供了有效的化疗效果。在双重刺激(近红外(NIR)光和肿瘤微环境中典型的酸性 pH 值)的触发下,DOX 在肿瘤部位按需释放,专门针对癌细胞,从而减轻了全身副作用。这些独特的特性有助于在体外和体内有效根除骨肉瘤。此外,该支架的组成模拟了天然骨骼的矿物相,并通过 PDA 的生物相容性得到了增强,从而促进了关键的成骨和血管生成过程。这不仅有利于消除肿瘤,还有利于健康骨组织的再生。总之,这项研究为骨肉瘤引起的骨缺损再生提供了一种有效的候选物质。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


