A novel mixed depressant for the flotation separation of scheelite and cassiterite: Adsorption mechanism and performance
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
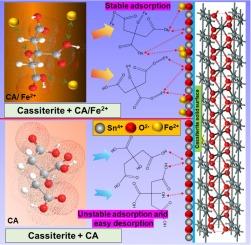
Citric acid (CA) exhibits weak inhibitory on some oxidized gangue minerals owing to its relatively few hydrophilic polar groups (![]() OH and
OH and ![]() COOH). This study investigate the synergistic mechanism of ferrous ions (Fe2+) to enhance both the selective adsorption capacity and stability of CA. The separation of scheelite and cassiterite through a flotation process was successfully achieved using a Fe2+/CA mixed depressant. Micro-flotation test results revealed that with the addition of Fe2+/CA, 84.60 % of scheelite and 23.64 % of cassiterite were recovered in the foam concentrate, with grades of 66.26 % and 17.73 %, respectively. Fe2+/CA had a strong depressing effect on cassiterite, resulting in a 69.30 % decrease in its recovery and a 20.33 % decrease in its grade. This indicates the effective separation of scheelite from cassiterite. Fe2+/CA exhibited a high adsorption affinity for the cassiterite surface, which mainly comprised L3–, Fe2+, and FeOH+ components. Additionally, the FeOH+ complex, formed from the pre-reaction between Fe2+ and CA, exhibited significant chemical adsorption at the Sn active sites, thereby depressing cassiterite. The high-intensity adsorption peaks at cracks and the significant increase in the normalized intensity of FeOH+ on the cassiterite surface indicated that the FeOH+ complex had stronger adsorption on cassiterite and selectively depressed cassiterite. This enhanced both the selective adsorption capacity and stability of CA on the cassiterite surface. The Fe2+/CA mixed depressant achieved efficient flotation separation of scheelite and cassiterite.
COOH). This study investigate the synergistic mechanism of ferrous ions (Fe2+) to enhance both the selective adsorption capacity and stability of CA. The separation of scheelite and cassiterite through a flotation process was successfully achieved using a Fe2+/CA mixed depressant. Micro-flotation test results revealed that with the addition of Fe2+/CA, 84.60 % of scheelite and 23.64 % of cassiterite were recovered in the foam concentrate, with grades of 66.26 % and 17.73 %, respectively. Fe2+/CA had a strong depressing effect on cassiterite, resulting in a 69.30 % decrease in its recovery and a 20.33 % decrease in its grade. This indicates the effective separation of scheelite from cassiterite. Fe2+/CA exhibited a high adsorption affinity for the cassiterite surface, which mainly comprised L3–, Fe2+, and FeOH+ components. Additionally, the FeOH+ complex, formed from the pre-reaction between Fe2+ and CA, exhibited significant chemical adsorption at the Sn active sites, thereby depressing cassiterite. The high-intensity adsorption peaks at cracks and the significant increase in the normalized intensity of FeOH+ on the cassiterite surface indicated that the FeOH+ complex had stronger adsorption on cassiterite and selectively depressed cassiterite. This enhanced both the selective adsorption capacity and stability of CA on the cassiterite surface. The Fe2+/CA mixed depressant achieved efficient flotation separation of scheelite and cassiterite.
用于浮选分离白钨矿和锡石的新型混合抑制剂:吸附机理和性能
柠檬酸(CA)由于亲水性极性基团(OH 和 COOH)相对较少,对一些氧化煤矸石矿物具有较弱的抑制作用。本研究探讨了亚铁离子(Fe2+)增强 CA 选择性吸附能力和稳定性的协同机制。使用 Fe2+/CA 混合抑制剂,通过浮选工艺成功实现了白钨矿和锡石的分离。微浮选试验结果表明,加入 Fe2+/CA 后,泡沫精矿中回收了 84.60 % 的白钨矿和 23.64 % 的锡石,品位分别为 66.26 % 和 17.73 %。Fe2+/CA 对锡石有强烈的抑制作用,导致其回收率下降 69.30%,品位下降 20.33%。这表明白钨矿能从锡石中有效分离出来。Fe2+/CA 对锡石表面具有很高的吸附亲和力,主要由 L3-、Fe2+ 和 FeOH+ 组成。此外,Fe2+ 和 CA 预反应形成的 FeOH+ 复合物在锡活性位点上有明显的化学吸附作用,从而抑制锡石。裂缝处的高强度吸附峰和锡石表面上 FeOH+ 的归一化强度的显著增加表明,FeOH+ 复合物对锡石有更强的吸附力,并能选择性地抑制锡石。这增强了 CA 在锡石表面的选择性吸附能力和稳定性。Fe2+/CA混合抑制剂实现了白钨矿和锡石的高效浮选分离。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


