The anisotropy of molybdenite planes: Analysis based on the adsorption behaviors of reagent and H2O
IF 4.4
2区 物理与天体物理
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Multiple planes with different properties are exposed after the comminution of molybdenite because of the anisotropy of molybdenite. Study on the properties of different planes is of great significance to enhance the comprehensive utilization of molybdenite, especially in flotation. In order to explore the properties of different planes, the two main planes of molybdenite (basal plane and edge plane) are respectively taken as the research object in this paper, and the properties of planes are expressed using DFT through the adsorption behaviors of a new reagent named DTC-CTS and water molecules. The results show that edge plane has higher activity than basal plane. This is because there are exposed molybdenum atoms on the edge plane, and the sulfur atoms in DTC-CTS and oxygen atoms in water molecules are easy to react with the exposed molybdenum atoms. While the activity of basal plane is low because of the hinderance effect of sulfur atoms in the skin layer. The different adsorption behaviors of water molecules on molybdenite planes verified the hydrophobicity of different planes. In addition, the results also show that the water can affect the DTC-CTS adsorption on edge plane. Water can enhance interaction between single-bond sulfur atom and molybdenum atom, and weaken the interaction between double-bond sulfur atom and molybdenum atom on edge plane.
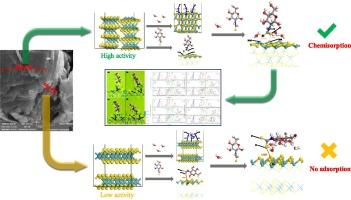
辉钼矿平面的各向异性:基于试剂和 H2O 吸附行为的分析
由于辉钼矿的各向异性,辉钼矿粉碎后会出现多种不同性质的平面。研究不同平面的性质对提高辉钼矿的综合利用,特别是在浮选中的应用具有重要意义。为了探讨不同平面的性质,本文分别以辉钼矿的两个主要平面(基底面和边缘面)为研究对象,通过一种名为 DTC-CTS 的新型试剂与水分子的吸附行为,利用 DFT 表达了平面的性质。结果表明,边缘平面比基底平面具有更高的活性。这是因为边缘平面上有裸露的钼原子,DTC-CTS 中的硫原子和水分子中的氧原子容易与裸露的钼原子发生反应。而由于表皮层中硫原子的阻碍作用,基底面的活性较低。水分子在辉钼矿平面上的不同吸附行为验证了不同平面的疏水性。此外,研究结果还表明,水会影响 DTC-CTS 在边缘平面上的吸附。水可以增强单键硫原子和钼原子之间的相互作用,削弱边缘平面上双键硫原子和钼原子之间的相互作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Results in Physics
MATERIALS SCIENCE, MULTIDISCIPLINARYPHYSIC-PHYSICS, MULTIDISCIPLINARY
CiteScore
8.70
自引率
9.40%
发文量
754
审稿时长
50 days
期刊介绍:
Results in Physics is an open access journal offering authors the opportunity to publish in all fundamental and interdisciplinary areas of physics, materials science, and applied physics. Papers of a theoretical, computational, and experimental nature are all welcome. Results in Physics accepts papers that are scientifically sound, technically correct and provide valuable new knowledge to the physics community. Topics such as three-dimensional flow and magnetohydrodynamics are not within the scope of Results in Physics.
Results in Physics welcomes three types of papers:
1. Full research papers
2. Microarticles: very short papers, no longer than two pages. They may consist of a single, but well-described piece of information, such as:
- Data and/or a plot plus a description
- Description of a new method or instrumentation
- Negative results
- Concept or design study
3. Letters to the Editor: Letters discussing a recent article published in Results in Physics are welcome. These are objective, constructive, or educational critiques of papers published in Results in Physics. Accepted letters will be sent to the author of the original paper for a response. Each letter and response is published together. Letters should be received within 8 weeks of the article''s publication. They should not exceed 750 words of text and 10 references.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


