Porous and defective NiCo2O4 spinel derived from bimetallic NiCo-based Prussian blue analogue for enhanced hydrogen production via the urea electro-oxidation reaction
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
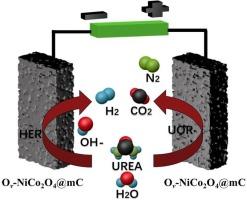
The efficient hydrogen production via overall water splitting is seriously restrained by the slow kinetics of the oxygen evolution reaction (OER). The substantially low potential for the urea electro-oxidation reaction (UOR) can tackle this problem by replacing the OER at the electrolyzer anode. Herein, the NiCo2O4 spinel with rich oxygen vacancies (Ov) and embedded within mesoporous carbon network (Ov-NiCo2O4@mC) was derived from NiCo-based Prussian blue analogous (NiCo PBA) and exploited as the bifunctional electrocatalyst of the UOR and OER for boost the hydrogen production via the overall water splitting. Benefiting to the regular skeleton and abundant dual metal sites of NiCo PBA, the derived Ov-NiCo2O4@mC comprises abundant oxygen vacancies, lattice defects, and adjustable electron structure. These features afford Ov-NiCo2O4@mC the improved UOR ability, showing the potential of 1.33 V versus reversible hydrogen electrode (RHE) at the current density of 10 mA cm−2, along with a small Tafel slopes of 31 mV dec−1, remarkably lower than that of the OER (1.51 V versus RHE, Tafel slope = 85 mV dec−1). The UOR performance of Ov-NiCo2O4@mC substantially outperforms to most of the reported Co and/or Ni-based electrocatalysts. Impressively, the assembled two-electrode urea-assisted overall water splitting device shows the small voltage for the hydrogen production (1.43 V versus RHE) and good long-term stability. The present work can provide a new alternative for the efficient hydrogen production using the MOFs-derivatives.
双金属镍钴基普鲁士蓝类似物衍生的多孔和缺陷镍钴氧化物尖晶石通过尿素电氧化反应提高制氢能力
由于氧进化反应(OER)的动力学速度较慢,通过整体水分裂高效制氢受到严重制约。尿素电氧化反应(UOR)的电位很低,可以通过在电解槽阳极取代 OER 来解决这一问题。在此,从镍钴基普鲁士蓝类似物(NiCo PBA)中衍生出了具有丰富氧空位(Ov)并嵌入介孔碳网络的镍钴氧化物尖晶石(Ov-NiCo2O4@mC),并将其用作尿素电氧化反应和尿素电还原反应的双功能电催化剂,通过整体水分离提高氢气产量。得益于镍钴铅蓝的规则骨架和丰富的双金属位点,衍生出的 Ov-NiCo2O4@mC 具有丰富的氧空位、晶格缺陷和可调电子结构。在 10 mA cm-2 的电流密度下,Ov-NiCo2O4@mC 相对于可逆氢电极(RHE)的电位为 1.33 V,塔菲尔斜率为 31 mV dec-1,明显低于 OER(相对于 RHE 的电位为 1.51 V,塔菲尔斜率 = 85 mV dec-1)。Ov-NiCo2O4@mC 的 UOR 性能大大优于大多数已报道的 Co 和/或 Ni 基电催化剂。令人印象深刻的是,所组装的双电极脲辅助整体水分离装置显示出较小的制氢电压(相对于 RHE 为 1.43 V)和良好的长期稳定性。本研究为利用 MOFs 衍生物高效制氢提供了一种新的选择。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


