Targeted regulation of autophagy using sorafenib-loaded biomineralization nanoenzyme for enhanced photodynamic therapy of hepatoma
IF 8.7
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Sorafenib (SF), a multi-targeted tyrosine kinase inhibitor, serves as a primary therapeutic modality for advanced liver cancer. Nonetheless, its clinical efficacy is hindered by various obstacles, such as limited bioavailability and inadequate accumulation. This study introduces a novel biomimetic mineralization enzyme, known as BSA@Pt/Ce6/SF@M (PCFM). The PCFM incorporates platinum (Pt) as a catalytic agent, SF as a molecular-targeted therapeutic agent, and Ce6 as a photosensitizer within liver cancer cell membranes. This strategy enables the combination of various anti-tumor treatments, such as photodynamic therapy (PDT) and autophagy induction, leading to increased bioavailability of SF and achieving a multidimensional synergistic anticancer effect. The PDT effect produced by Ce6 in PCFM greatly enhances SF-induced autophagy, effectively promoting autophagic cell death. Furthermore, Pt dissociates from the biomineralization process, acquiring peroxidase properties through chemokinetic reactions. This facilitates the catalysis of significant oxygen generation, addressing the challenge of hypoxia in the tumor microenvironment and improving the efficacy of PDT. Moreover, the SF further enhances therapeutic efficacy by inducing autophagy in response to energy deprivation, as indicated by the reduced levels of HIF-1α, p62, along with increased levels of ROS and LC3-Ⅱ/Ι. This biomineralization-based nanoenzyme exhibits strong anti-tumor characteristics, offering a novel strategy for overcoming challenges in liver cancer treatment.
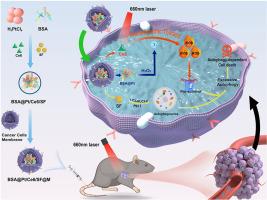
利用装载索拉非尼的生物矿化纳米酶靶向调节自噬,增强肝癌的光动力疗法
索拉非尼(Sorafenib)是一种多靶点酪氨酸激酶抑制剂,是晚期肝癌的主要治疗方法。然而,其临床疗效却受到生物利用度有限和蓄积不足等各种障碍的阻碍。本研究介绍了一种新型仿生矿化酶,即 BSA@Pt/Ce6/SF@M(PCFM)。PCFM 将铂 (Pt) 作为催化剂、SF 作为分子靶向治疗剂以及 Ce6 作为光敏剂结合到肝癌细胞膜中。这种策略能够将光动力疗法(PDT)和自噬诱导等多种抗肿瘤疗法结合起来,从而提高 SF 的生物利用率,实现多维协同抗癌效果。PCFM 中的 Ce6 产生的光动力疗法效应大大增强了 SF 诱导的自噬作用,有效促进了细胞的自噬死亡。此外,铂在生物矿化过程中解离,通过趋化反应获得过氧化物酶特性。这有利于催化大量氧气的生成,从而解决肿瘤微环境缺氧的难题,提高光动力疗法的疗效。此外,SF 还能通过诱导自噬来响应能量剥夺,从而进一步提高疗效,HIF-1α 和 p62 水平的降低以及 ROS 和 LC3-Ⅱ/Ι 水平的升高就说明了这一点。这种基于生物矿化的纳米酶具有很强的抗肿瘤特性,为克服肝癌治疗难题提供了一种新策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
文献相关原料
公司名称
产品信息
阿拉丁
Ce6
阿拉丁
Chloroplatinic acid
阿拉丁
BSA
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


