Flupyradifurone activates DUM neuron nicotinic acetylcholine receptors and stimulates an increase in intracellular calcium through the ryanodine receptors
IF 4.2
1区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Insect neuronal nicotinic acetylcholine receptors (nAChRs) are transmembrane receptors that play a key role in the development and synaptic plasticity of both vertebrates and invertebrates, and are considered to be major targets of several insecticides. We used dorsal unpaired median (DUM) neurons, which are insect neurosecretory cells, to explore what type of nAChRs are involved in flupyradifurone's (FLU) mode of action, and to study the role of calcium release from intracellular stores in this process. Using whole-cell patch-clamp and fura-2-AM calcium imaging techniques, we found that inhibition of IP3Rs through application of 2-APB reduced FLU inward currents, but did not affect the intracellular calcium release induced by FLU. In contrast, inhibition of RyRs using ryanodine, led to reduction of intracellular calcium increase following FLU pulse application. These results suggested that FLU inward currents are likely due to a combination of the direct effects of FLU on DUM neuron nAChRs and the subsequent calcium release from RyRs.
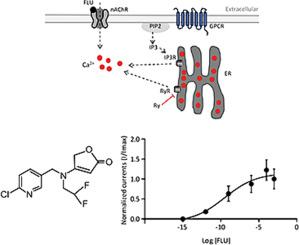
氟吡拉呋酮可激活 DUM 神经元的烟碱乙酰胆碱受体,并通过雷诺丁受体刺激细胞内钙的增加
昆虫神经元烟碱乙酰胆碱受体(nAChRs)是一种跨膜受体,在脊椎动物和无脊椎动物的发育和突触可塑性中起着关键作用,被认为是多种杀虫剂的主要靶标。我们利用昆虫的神经分泌细胞--背侧无对正中神经元(DUM)来探索哪种类型的nAChRs参与了氟哌啶酮(FLU)的作用模式,并研究钙从细胞内储存释放在这一过程中的作用。通过使用全细胞膜片钳和呋喃-2-AM 钙成像技术,我们发现通过应用 2-APB 抑制 IP3Rs 可降低 FLU 的内向电流,但不会影响 FLU 诱导的细胞内钙释放。相反,使用雷诺丁抑制 RyRs 可减少 FLU 脉冲应用后细胞内钙的增加。这些结果表明,FLU 内向电流可能是 FLU 对 DUM 神经元 nAChRs 的直接作用和 RyRs 随后的钙释放共同作用的结果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.00
自引率
8.50%
发文量
238
审稿时长
4.2 months
期刊介绍:
Pesticide Biochemistry and Physiology publishes original scientific articles pertaining to the mode of action of plant protection agents such as insecticides, fungicides, herbicides, and similar compounds, including nonlethal pest control agents, biosynthesis of pheromones, hormones, and plant resistance agents. Manuscripts may include a biochemical, physiological, or molecular study for an understanding of comparative toxicology or selective toxicity of both target and nontarget organisms. Particular interest will be given to studies on the molecular biology of pest control, toxicology, and pesticide resistance.
Research Areas Emphasized Include the Biochemistry and Physiology of:
• Comparative toxicity
• Mode of action
• Pathophysiology
• Plant growth regulators
• Resistance
• Other effects of pesticides on both parasites and hosts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


