New advances in the enhancement of direct mercury speciation in solid matter on the example of HgCl, CH3HgCl, HgS and HgSO4 using programmable thermal release in combination with electrothermal atomic absorption spectrometry
IF 4.9
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Mercury is considering as hazardous pollutant presented as a number of various species characterized by different migration routes, bioavailability and toxicity. In this regard, the characterization of natural and a fortiori anthropogenic environment by the concentration of various chemical forms of mercury is relevant to assess the degree of its impact on living organisms. From this point of view, solid samples are the most problematic ones due to the need for preliminary extraction of analytes into solution. The promising approach for this purpose is analysis based on a combination of thermal release as separation way with sequential electrothermal atomic absorption spectrometric detection (TR-ETA-AAS). The present work is based on research of the thermal behavior of individual mercury species in argon atmosphere when the evaporation and atomization zones are separated and a programmable heating algorithm is implemented. To unify an analytical procedure the dilution of reference and analyzed samples with aluminum oxide was applied. The developed method allows determination of the species most often found in natural and contaminated environments: mercury (II) chloride, methylmercury chloride, mercury sulfide and sulphate at the level of 0.003–0.030 µg with a reproducibility of 10–25 %,. The trueness of the proposed approach has been confirmed using certified reference material ERM CE464 and spiking method.
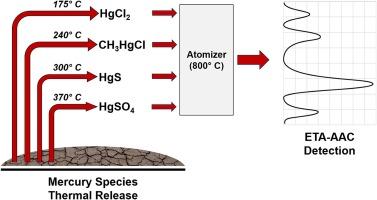
以 HgCl、CH3HgCl、HgS 和 HgSO4 为例,利用可编程热释放技术结合电热原子吸收光谱法,在提高固体物质中的直接汞标示方面取得新进展
汞被认为是一种危险的污染物,它有许多不同的种类,具有不同的迁移路径、生物利用率和毒性。因此,通过各种化学形式的汞浓度来描述自然环境和人为环境的特征,对于评估汞对生物体的影响程度非常重要。从这个角度来看,固体样本最容易出现问题,因为需要将分析物初步提取到溶液中。为此,最有前途的方法是将热释放作为分离方式与顺序电热原子吸收光谱检测(TR-ETA-AAS)相结合进行分析。当蒸发区和雾化区分离并采用可编程加热算法时,本研究工作基于对氩气环境中单个汞物种热行为的研究。为了统一分析程序,采用了氧化铝稀释参考样品和分析样品的方法。所开发的方法可以在 0.003-0.030 µg 的水平上测定自然环境和污染环境中最常见的物种:氯化汞 (II)、氯化甲基汞、硫化汞和硫酸盐,重现性为 10-25%。使用认证参考材料 ERM CE464 和加标法确认了拟议方法的准确性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microchemical Journal
化学-分析化学
CiteScore
8.70
自引率
8.30%
发文量
1131
审稿时长
1.9 months
期刊介绍:
The Microchemical Journal is a peer reviewed journal devoted to all aspects and phases of analytical chemistry and chemical analysis. The Microchemical Journal publishes articles which are at the forefront of modern analytical chemistry and cover innovations in the techniques to the finest possible limits. This includes fundamental aspects, instrumentation, new developments, innovative and novel methods and applications including environmental and clinical field.
Traditional classical analytical methods such as spectrophotometry and titrimetry as well as established instrumentation methods such as flame and graphite furnace atomic absorption spectrometry, gas chromatography, and modified glassy or carbon electrode electrochemical methods will be considered, provided they show significant improvements and novelty compared to the established methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


