Development of a new rhodamine 6G based probe and its application as an optical sensor of Cu2+ and Fe3+ ions: A comprehensive experimental and Theoretical studies
IF 4.9
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
A new rhodamine appended probe 3-allyl salicylaldehyde rhodamine hydrazone (RGAL) has been synthesized and thoroughly characterized using various spectroscopic techniques, as well as single crystal XRD. The optical properties of RGAL were investigated in 10 mM HEPES buffer in H2O:CH3CN (2:8, v/v, pH=7.2) in the presence of various cations. RGAL showed selectivity and sensitivity towards Cu2+ during absorption process and “turn on” behavior towards Fe3+ during emission study owing to the opening of a spirolactum ring. The detection limits for Cu2+ and Fe3+ ions using RGAL were determined to be 6.15 ppm and 4.75 ppm, respectively. The binding constant of RGAL with Cu2+ and Fe3+ ions was found to be 1.20 × 104 M−1 and 1.71 × 104 M−1, respectively. Hirshfeld surface and fingerprint analysis of RGAL provides the in-depth analysis of pairwise interaction between two atoms. Furthermore, the topological analysis of RGAL is performed using NCI, AIM, ELF and LOL analysis. The analysis provides information about O78-H79…N71 and C40-H41…O77 hydrogen bonding interactions in the monomer of RGAL whereas various inter- and intra- molecular interactions give strength to the dimer pattern of RGAL.
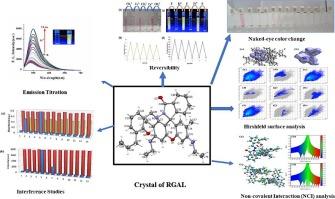
基于罗丹明 6G 的新型探针的开发及其作为 Cu2+ 和 Fe3+ 离子光学传感器的应用:综合实验与理论研究
我们合成了一种新的罗丹明附加探针 3-烯丙基水杨醛罗丹明腙(RGAL),并利用各种光谱技术和单晶 XRD 对其进行了全面表征。在 10 mM HEPES 缓冲溶液(H2O:CH3CN(2:8, v/v, pH=7.2))中,在各种阳离子的存在下,研究了 RGAL 的光学特性。在吸收过程中,RGAL 表现出对 Cu2+ 的选择性和灵敏度;在发射研究中,由于螺内酯环的打开,RGAL 表现出对 Fe3+ 的 "开启 "行为。使用 RGAL 对 Cu2+ 和 Fe3+ 离子的检测限分别为 6.15 ppm 和 4.75 ppm。RGAL 与 Cu2+ 和 Fe3+ 离子的结合常数分别为 1.20 × 104 M-1 和 1.71 × 104 M-1。RGAL 的 Hirshfeld 表面和指纹分析深入分析了两个原子之间的配对相互作用。此外,还利用 NCI、AIM、ELF 和 LOL 分析法对 RGAL 进行了拓扑分析。该分析提供了 RGAL 单体中 O78-H79...N71 和 C40-H41...O77 氢键相互作用的信息,而各种分子间和分子内相互作用则为 RGAL 的二聚体模式提供了强度。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microchemical Journal
化学-分析化学
CiteScore
8.70
自引率
8.30%
发文量
1131
审稿时长
1.9 months
期刊介绍:
The Microchemical Journal is a peer reviewed journal devoted to all aspects and phases of analytical chemistry and chemical analysis. The Microchemical Journal publishes articles which are at the forefront of modern analytical chemistry and cover innovations in the techniques to the finest possible limits. This includes fundamental aspects, instrumentation, new developments, innovative and novel methods and applications including environmental and clinical field.
Traditional classical analytical methods such as spectrophotometry and titrimetry as well as established instrumentation methods such as flame and graphite furnace atomic absorption spectrometry, gas chromatography, and modified glassy or carbon electrode electrochemical methods will be considered, provided they show significant improvements and novelty compared to the established methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


