Photocatalytic water splitting for hydrogen and oxygen evolution using cobalt- substituted polyoxometalates
IF 2.5
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The synthesis and photocatalytic properties of cobalt-incorporated polyoxometalate H18[α1-P2W17Co(H2O)O61]-[Co4(H2O)2(PW9O34)2] (1) were reported, and split into α1-H8-[P2W17Co(H2O)O61] (2) and H10[Co4(H2O)2(PW9O34)2] (3) by adjusting the types of acids and solvents in the reaction. The photocatalytic production of O2 and H2 from water by compounds 1, 2 and 3 was evaluated. Notably, compounds 1 and 2 demonstrate superior performance in the photocatalytic production of both hydrogen and oxygen, whereas compound 3 solely exhibits activity in the photocatalytic generation of oxygen. The significantly enhanced performance against the control [P2W18O62]6− can be attributed to the modulation of the electronic structures of these novel POMs facilitated by the incorporation of cobalt.
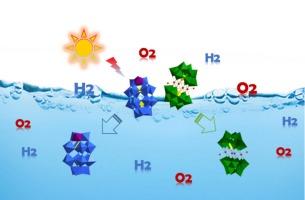
使用钴取代的多氧金属酸盐进行光催化水分离以实现氢和氧的进化
报告了掺钴聚氧化金属酸盐 H18[α1-P2W17Co(H2O)O61]-[Co4(H2O)2(PW9O34)2](1)的合成和光催化性能,并通过调整反应中酸和溶剂的种类将其分为 α1-H8-[P2W17Co(H2O)O61](2)和 H10[Co4(H2O)2(PW9O34)2](3)。评估了化合物 1、2 和 3 从水中光催化产生 O2 和 H2 的情况。值得注意的是,化合物 1 和 2 在光催化产生氢气和氧气方面表现出卓越的性能,而化合物 3 仅在光催化产生氧气方面表现出活性。与对照[P2W18O62]6-相比,化合物 1 和化合物 2 的性能明显增强,这是因为钴的加入改变了这些新型 POM 的电子结构。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


