Rational design, synthesis and anticancer screening of 1,2,4-oxadiazole incorporated thieno[2,3-d]thiazole-isoxazole-pyridine derivatives
IF 2.5
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
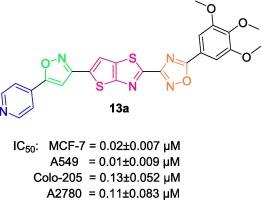
We have design and synthesized a new series of 1,2,4-oxadiazole incorporated thieno[2,3-d]thiazole-isoxazole-pyridine analogues (13a-j) and were confirmed by 1H NMR, 13C NMR and mass spectral data. Further, the newly synthesized compounds (13a–j) were evaluated for their preliminary anticancer activity against a panel of four human cancer cell lines such as MCF-7 (breast cancer), A549 (lung cancer), Colo-205 (colon cancer) & A2780 (ovarian cancer) by using of MTT method, the known chemotherapeutic agent as etoposide used as positive control. Most of the tested compounds were displayed good to moderate activities on all cell lines. The IC50 values showed compound ranges from 0.01 ± 0.009 µM to 8.41 ± 5.48 µM. Where positive control showed values range from 3.34 ± 0.152 µM to 0.17 ± 0.034 µM. Among them, compound 13a, 13b, 13c, 13d, 13e and 13f were showed more potent activity than etoposide. Predominantly, the compound 13a showed potent anticancer activity against MCF-7, A549, Colo-205, and A2780 cancer cell lines with IC50 values of 0.02 ± 0.007 µM, 0.01 ± 0.009 µM, 0.13 ± 0.052 µM, and 0.11 ± 0.083 µM.
1,2,4-噁二唑与噻吩并[2,3-d]噻唑-异噁唑-吡啶衍生物的合理设计、合成和抗癌筛选
我们设计并合成了一系列新的 1,2,4-噁二唑并噻吩并[2,3-d]噻唑-异噁唑-吡啶类似物(13a-j),并通过 1H、13C NMR 和质谱数据进行了证实。此外,还采用 MTT 法评估了新合成的化合物(13a-j)对四种人类癌症细胞系(如 MCF-7(乳腺癌)、A549(肺癌)、Colo-205(结肠癌)和amp;A2780(卵巢癌))的初步抗癌活性,并以已知的化疗药物依托泊苷作为阳性对照。大多数受试化合物对所有细胞系都显示出良好至中等程度的活性。化合物的 IC50 值从 0.01 ± 0.009 µM 到 8.41 ± 5.48 µM。阳性对照的 IC50 值为 3.34 ± 0.152 µM 至 0.17 ± 0.034 µM。其中,化合物 13a、13b、13c、13d、13e 和 13f 比依托泊苷显示出更强的活性。主要是化合物 13a 对 MCF-7、A549、Colo-205 和 A2780 癌细胞株显示出了强大的抗癌活性,其 IC50 值分别为 0.02 ± 0.007 µM、0.01 ± 0.009 µM、0.13 ± 0.052 µM 和 0.11 ± 0.083 µM。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


