Laboratory considerations in the assessment of 25-hydroxyvitamin D in pregnant women by automated immunoassays
IF 1.7
Q3 MEDICAL LABORATORY TECHNOLOGY
引用次数: 0
Abstract
Background
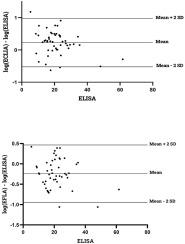
Because of the pathophysiological role of vitamin D in health, there is an increased interest to check the clinical status of this vitamin. Immunochemical assays are commonly employed to determine 25-hydroxyvitamin D (25 (OH) D) in clinical laboratories and its testing could be influenced by pre-analytic and analytic issues. The aim of this study was to compare the 25(OH)D results obtained from three commonly used immunoassays in pregnant women to check a possible discrepancy between tests.
Material and methods
A group of 50 pregnant women who were in their third trimester were included in this study. The quantification of serum vitamin D was performed utilizing three immunochemistry-based assays including Elecsys, VIDAS and Alegria. We also involved 21 non-pregnant volunteers to clinically assess the vitamin D status in this group of people.
Results
Our findings revealed a significant inconsistency between the obtained results from three assays for serum 25(OH)D. The 25(OH)D showed higher values when measured by the Elecsys assay while the VIDAS assay had lower values compared to the other immunoassays. More notably, the 25(OH)D testing in non-pregnant subjects showed consistent results in all three immunoassays.
Conclusions
The results of the 25(OH)D measurements in pregnant women should be interpreted carefully due to a great inaccuracy in immunoassay testing. There is no such disagreement in non-pregnant people. Standardization of vitamin D testing in various settings is a crucial matter for clinical laboratories.
使用自动免疫测定法评估孕妇体内 25- 羟维生素 D 的实验室注意事项
背景由于维生素 D 在健康中的病理生理作用,人们对检查这种维生素的临床状态越来越感兴趣。临床实验室通常采用免疫化学方法来测定 25- 羟基维生素 D(25 (OH) D),其检测结果可能会受到分析前和分析过程中问题的影响。本研究的目的是比较三种常用免疫测定法测定孕妇 25(OH)D 的结果,以检查不同检测方法之间可能存在的差异。使用三种基于免疫化学的检测方法(包括 Elecsys、VIDAS 和 Alegria)对血清维生素 D 进行定量。我们还邀请了 21 名非孕妇志愿者对这部分人群的维生素 D 状态进行临床评估。与其他免疫测定法相比,Elecsys 法测定的 25(OH)D 值较高,而 VIDAS 法测定的 25(OH)D 值较低。更值得注意的是,在对非孕妇进行 25(OH)D 检测时,所有三种免疫测定法的结果都是一致的。在非孕妇中则没有这种分歧。对临床实验室来说,在不同环境中进行维生素 D 检测的标准化至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Practical Laboratory Medicine
Health Professions-Radiological and Ultrasound Technology
CiteScore
3.50
自引率
0.00%
发文量
40
审稿时长
7 weeks
期刊介绍:
Practical Laboratory Medicine is a high-quality, peer-reviewed, international open-access journal publishing original research, new methods and critical evaluations, case reports and short papers in the fields of clinical chemistry and laboratory medicine. The objective of the journal is to provide practical information of immediate relevance to workers in clinical laboratories. The primary scope of the journal covers clinical chemistry, hematology, molecular biology and genetics relevant to laboratory medicine, microbiology, immunology, therapeutic drug monitoring and toxicology, laboratory management and informatics. We welcome papers which describe critical evaluations of biomarkers and their role in the diagnosis and treatment of clinically significant disease, validation of commercial and in-house IVD methods, method comparisons, interference reports, the development of new reagents and reference materials, reference range studies and regulatory compliance reports. Manuscripts describing the development of new methods applicable to laboratory medicine (including point-of-care testing) are particularly encouraged, even if preliminary or small scale.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


