Transition metal nickel nanoparticle-decorated g-C3N4 photocatalyst for improving tetracycline hydrochloride degradation
IF 4.1
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2024-09-24
DOI:10.1016/j.jphotochem.2024.116042
引用次数: 0
Abstract
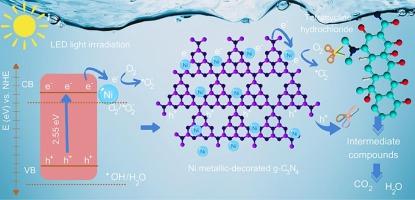
Metallic nickel nanoparticles (Ni0 NPs)-supported g-C3N4 have been developed using a solvothermal technique in N,N-dimethylformamide solvent. A uniformly dispersion of Ni0 NPs anchored on a g-C3N4 photocatalyst can improve the photocatalytic efficiency. The optimized Ni/g-C3N4 catalyst demonstrated a high photodegradation rate of TCH at 6.0 × 10−3 min−1 under LED light irradiation, which was approximately three times greater than that of the bare g-C3N4 catalyst. This superior performance originates from the capable separation of electron-hole pairs, facilitated by the strong interaction between Ni0 NPs and the host g-C3N4, as supported by optical and electrochemical property analysis. Our work provides a facile manner for the effective degradation of pollutants using heterojunction photocatalysts.
用于改善盐酸四环素降解的过渡金属镍纳米颗粒装饰 g-C3N4 光催化剂
在 N,N-二甲基甲酰胺溶剂中利用溶热技术开发了金属镍纳米粒子(Ni0 NPs)支撑的 g-C3N4。在 g-C3N4 光催化剂上均匀分散的 Ni0 NPs 可以提高光催化效率。优化后的 Ni/g-C3N4 催化剂在 LED 光照射下对 TCH 的光降解率高达 6.0 × 10-3 min-1,约为裸 g-C3N4 催化剂的三倍。这一优异性能源于 Ni0 NPs 与宿主 g-C3N4 之间的强相互作用促进了电子-空穴对的分离,光学和电化学性能分析也证明了这一点。我们的工作为利用异质结光催化剂有效降解污染物提供了一种简便的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


