A MAUDE database analysis on the new generation of active bone conduction hearing implants
Abstract
Objective
To analyze medical device reports (MDR) submitted to the Food and Drug Administration's (FDA) Manufacturer and User Device Facility Experience (MAUDE) database to identify adverse events (AEs) in patients implanted with novel active bone conduction hearing implants (BCIs).
Methods
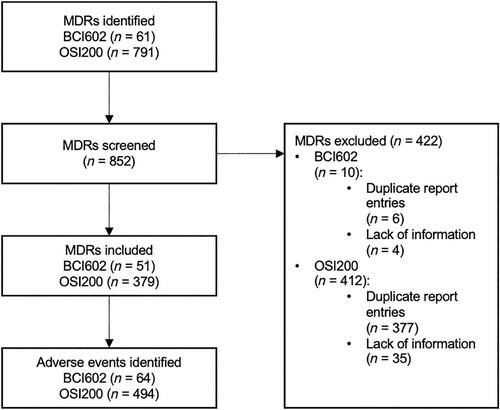
We conducted a search of the FDA MAUDE database on the newest generation of BCIs. Data were collected concerning device malfunctions, patient injuries, factors triggering these incidents, and the subsequent actions taken.
Results
In total, 93 (16.7%) device malfunctions and 465 (83.3%) patient injuries with 358 subsequent interventions were identified, resulting in 558 AEs. Although the absolute AE number per device cannot be identified, the following trends were detected: Among the 494 AEs associated with OSI200, 55 (11.1%) reported device malfunctions and 454 (88.9%) cited patient injuries. Out of the 64 AEs linked to BCI602, 28 (59.4%) were associated with malfunctions, whereas 26 (40.6%) involved patient injuries. The most frequently reported particular AEs for the OSI200 were infection (n = 171, 34.6%), extrusion of the device (n = 107, 21.7%), and pain (n = 51, 10.3%). Conversely, no device output (n = 20, 31.3%) and loss of osseointegration (n = 7, 10.9%) were the most reported AEs for the BCI602. Various AEs led to 214 explanations and 77 revision surgeries. Sixty-seven AEs reported conservative treatment.
Conclusion
The current study provides an overview of the most commonly reported complications with new active BCIs. Although providing an overview, given the limitations of the FDA MAUDE database, our results have to be interpreted with caution.
Level of Evidence
4.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


