下载PDF
{"title":"RSVpreF vaccination in pregnancy: a meta-analysis of maternal-fetal safety and infant efficacy.","authors":"Greg J Marchand, Ahmed Taher Massoud, Ahmed Taha Abdelsattar, Peter A McCullough","doi":"10.5468/ogs.24213","DOIUrl":null,"url":null,"abstract":"<p><p>In May 2023, the United States Food and Drug Administration approved a Pfizer©-sponsored (Pfizer, New York, NY, USA) bivalent respiratory syncytial virus prefusion F protein-based vaccine (RSVpreF) RSV vaccine (AbrysvoTM [Pfizer]) for use during pregnancy to prevent neonatal/infant RSV infection. In February of 2022, trials sponsored by GSK© (Brentford, England, UK) on a similar RSVpreF vaccine were halted because of the identification of a safety signal related to preterm births. As these vaccines use identical pre-fusion F-protein technology, we sought to synthesize the existing data on their effectiveness and safety. We identified all randomized controlled trials and used RevMan 5.4.1 (The Cochrane Collaboration, England, UK) to perform the analysis with 95% confidence intervals and risk ratios (RRs). We found many maternal side effects were more prevalent in the RSVpreF group, with more local reactions, blood disorders, fatigue, joint pain, cardiac disorders, headache, fever, gastrointestinal disorders and pregnancy complications. The vaccinated group demonstrated significant reductions in RSV-lower respiratory tract cases (RR, 0.44 [0.33, 0.57]; P<0.00001), severe respiratory illness (RR, 0.29 [0.19, 0.44]; P<0.00001), and hospitalizations (RR, 0.40 [0.24, 0.67]; P=0.0005). RSVpreF vaccination was associated with a higher incidence of preterm delivery (RR, 1.24 [1.08, 1.44]; P=0.003). No significant difference in neonatal deaths was observed (RR, 1.42 [0.70, 2.89]; P=0.34). In conclusion, RSVpreF vaccination results in systemic adverse events and an increase in preterm delivery. Vaccination appears to have acceptable short-term newborn safety, but is not related to a significant decrease in neonatal death.</p>","PeriodicalId":37602,"journal":{"name":"Obstetrics and Gynecology Science","volume":" ","pages":"511-524"},"PeriodicalIF":1.9000,"publicationDate":"2024-11-01","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11581813/pdf/","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Obstetrics and Gynecology Science","FirstCategoryId":"1085","ListUrlMain":"https://doi.org/10.5468/ogs.24213","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"2024/9/27 0:00:00","PubModel":"Epub","JCR":"Q2","JCRName":"OBSTETRICS & GYNECOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
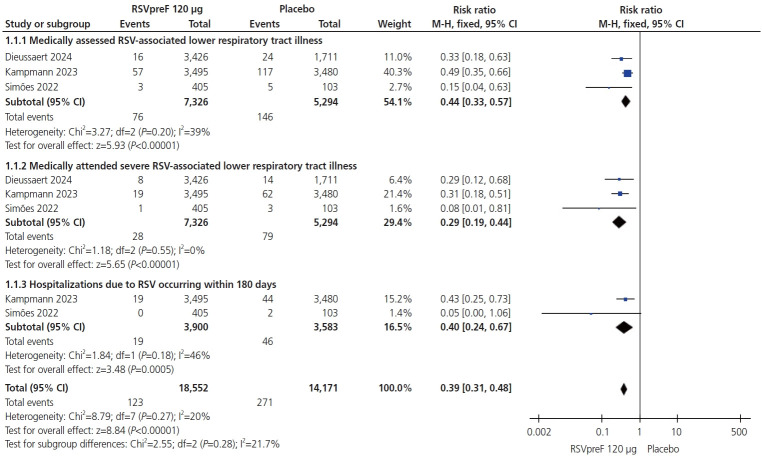
In May 2023, the United States Food and Drug Administration approved a Pfizer©-sponsored (Pfizer, New York, NY, USA) bivalent respiratory syncytial virus prefusion F protein-based vaccine (RSVpreF) RSV vaccine (AbrysvoTM [Pfizer]) for use during pregnancy to prevent neonatal/infant RSV infection. In February of 2022, trials sponsored by GSK© (Brentford, England, UK) on a similar RSVpreF vaccine were halted because of the identification of a safety signal related to preterm births. As these vaccines use identical pre-fusion F-protein technology, we sought to synthesize the existing data on their effectiveness and safety. We identified all randomized controlled trials and used RevMan 5.4.1 (The Cochrane Collaboration, England, UK) to perform the analysis with 95% confidence intervals and risk ratios (RRs). We found many maternal side effects were more prevalent in the RSVpreF group, with more local reactions, blood disorders, fatigue, joint pain, cardiac disorders, headache, fever, gastrointestinal disorders and pregnancy complications. The vaccinated group demonstrated significant reductions in RSV-lower respiratory tract cases (RR, 0.44 [0.33, 0.57]; P<0.00001), severe respiratory illness (RR, 0.29 [0.19, 0.44]; P<0.00001), and hospitalizations (RR, 0.40 [0.24, 0.67]; P=0.0005). RSVpreF vaccination was associated with a higher incidence of preterm delivery (RR, 1.24 [1.08, 1.44]; P=0.003). No significant difference in neonatal deaths was observed (RR, 1.42 [0.70, 2.89]; P=0.34). In conclusion, RSVpreF vaccination results in systemic adverse events and an increase in preterm delivery. Vaccination appears to have acceptable short-term newborn safety, but is not related to a significant decrease in neonatal death.
孕期 RSVpreF 疫苗接种:母胎安全性和婴儿疗效的荟萃分析。
2023 年 5 月,美国食品和药物管理局(FDA)批准辉瑞公司© 赞助的二价 RSVpreF(呼吸道合胞病毒前体 F 蛋白疫苗)RSV 疫苗(AbrysvoTM [公司、城市、州、国家])用于孕期预防新生儿/婴儿 RSV 感染。2022 年 2 月,FDA 停止了由葛兰素史克公司(GSK© [公司、城市、州、县])赞助的类似 RSVpreF RSV 疫苗的试验,原因是发现了与早产有关的安全信号。由于这些疫苗使用了几乎完全相同的前融合 F 蛋白技术,我们试图综合并评估有关其有效性和安全性的现有高质量文献。从开始到 2024 年 3 月 15 日,我们检索了有关这一主题的随机对照试验 (RCT)。我们使用Review Manager (RevMan 5.4.1)(公司、城市、州、国家)进行了95%置信区间和风险比(RR)分析。我们的搜索结果显示有三项大型 RCT。从安全性角度来看,RSVpreF 组的许多产妇副作用更大,局部反应(RR,5.98 [3.68, 6.83];P=0.01;I2=0%)、血液紊乱(RR,1.07 [0.69, 1.66];P=0.78;I2=0%)、疲劳(RR,1.05 [1.00, 1.10];P=0.07;I2=0%)、关节疼痛(RR,1.60, 1.39 [0.68,2.86];P=0.37;I2=59%)、心脏疾病(RR,1.19 [0.80,1.77];P=0.38;I2=0%)、头痛(RR,0.80 [0.30,2.10];P=0.65;I2=0%)、发热(RR,0.90 [0.20,4.16];P=0.89;I2=57%)、胃肠功能紊乱(RR,1.04 [0.70,1.56];P=0.83;I2=0%)和妊娠并发症(RR,1.01 [0.65,1.56];P=0.97;I2=17%)。接种疫苗组的 RSV 下呼吸道疾病(LRTD)病例(RR,0.44 [0.33,0.57];P=0.01)、严重下呼吸道疾病(RR,0.29 [0.19,0.44];P=0.01)和住院治疗(RR,0.40 [0.24,0.67];P=0.005)显著减少。接种 RSVpreF 疫苗与较高的早产发生率有关(RR,1.24 [1.08,1.44];P=0.03);但在新生儿死亡方面未观察到显著差异(RR,1.42 [0.70,2.89];P=0.34)。接种 RSVpreF 疫苗会导致全身性不良事件和早产率上升。接种疫苗似乎对新生儿具有可接受的短期安全性,但并没有显著减少新生儿死亡。
本文章由计算机程序翻译,如有差异,请以英文原文为准。

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


