Association of metabolic signatures of air pollution with MASLD: Observational and Mendelian randomization study
IF 33
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Background & Aims
Air pollution is a significant public health issue and an important risk factor for metabolic dysfunction-associated steatotic liver disease (MASLD), though the underlying mechanisms of this association are unknown. Herein, we aimed to identify metabolic signatures associated with exposure to ambient air pollution and to explore their associations with the risk of MASLD.
Methods
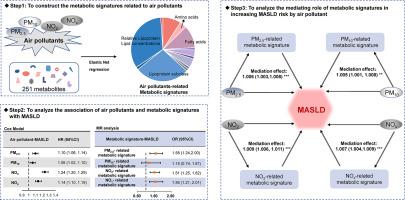
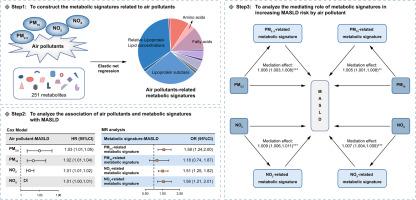
We utilized data from the UK Biobank cohort. Annual mean concentrations of PM2.5, PM10, NO2 and NOx were assessed for each participant using bilinear interpolation. The elastic net regression model was used to identify metabolites associated with four air pollutants and to construct metabolic signatures. Associations between air pollutants, metabolic signatures and MASLD were analyzed using Cox models. Mendelian randomization (MR) analysis was used to examine potential causality. Mediation analysis was employed to examine the role of metabolic signatures in the association between air pollutants and MASLD.
Results
A total of 244,842 participants from the UK Biobank were included in this analysis. We identified 87, 65, 76, and 71 metabolites as metabolic signatures of PM2.5, PM10, NO2, and NOx, respectively. Metabolic signatures were associated with risk of MASLD, with hazard ratios (HRs) and 95% CIs of 1.10 (1.06–1.14), 1.06 (1.02–1.10), 1.24 (1.20–1.29) and 1.14 (1.10–1.19), respectively. The four pollutants were associated with increased risk of MASLD, with HRs (95% CIs) of 1.03 (1.01–1.05), 1.02 (1.01–1.04), 1.01 (1.01–1.02) and 1.01 (1.00–1.01), respectively. MR analysis indicated an association between PM2.5, NO2 and NOx-related metabolic signatures and MASLD. Metabolic signatures mediated the association of PM2.5, PM10, NO2 and NOx with MASLD.
Conclusion
PM2.5, PM10, NO2 and NOx-related metabolic signatures appear to be associated with MASLD. These signatures mediated the increased risk of MASLD associated with PM2.5, PM10, NO2 and NOx.
Impact and implications
Air pollution is a significant public health issue and an important risk factor for metabolic dysfunction-associated steatotic liver disease (MASLD), however, the mechanism by which air pollution affects MASLD remains unclear. Our study used integrated serological metabolic data of 251 metabolites from a large-scale cohort study to demonstrate that metabolic signatures play a crucial role in the elevated risk of MASLD caused by air pollution. These results are relevant to patients and policymakers because they suggest that air pollution-related metabolic signatures are not only potentially associated with MASLD but also involved in mediating the process by which PM2.5, PM10, NO2, and NOx increase the risk of MASLD. Focusing on changes in air pollution-related metabolic signatures may offer a new perspective for preventing air pollution-induced MASLD and serve as protective measures to address this emerging public health challenge.
空气污染的代谢特征与 MASLD 的关系:观察和孟德尔随机研究。
背景与目的:确定与暴露于环境空气污染有关的代谢特征,并探讨其与代谢功能障碍相关性脂肪性肝病(MASLD)风险的关系:确定与暴露于环境空气污染有关的代谢特征,并探讨它们与代谢功能障碍相关性脂肪性肝病(MASLD)风险的关联:我们利用了英国生物库队列的数据。方法:我们利用英国生物库队列的数据,采用双线性内插法评估了每位参与者的 PM2.5、PM10、二氧化氮和氮氧化物的年平均浓度。使用弹性网回归模型分别确定了与四种空气污染物相关的代谢物,并构建了代谢特征。使用 Cox 模型分析了空气污染物、代谢特征和 MASLD 之间的关联。孟德尔随机化(MR)分析用于检验潜在的因果关系。采用中介分析法研究了代谢特征在空气污染物与 MASLD 之间的关联中的作用:共有 244 842 名来自英国生物库的参与者参与了此次分析。我们分别确定了87、65、76和71个代谢物作为PM2.5、PM10、二氧化氮和氮氧化物的代谢特征。代谢特征与 MASLD 风险相关,危险比(HRs)和 95% 置信区间(95% CIs)分别为 1.10(1.06,1.14)、1.06(1.02,1.10)、1.24(1.20,1.29)和 1.14(1.10,1.19)。这四种污染物与 MASLD 风险增加有关,HRs(95% CIs)分别为 1.03(1.01,1.05)、1.02(1.01,1.04)、1.01(1.01,1.02)和 1.01(1.00,1.01)。MR分析表明,PM2.5、二氧化氮和氮氧化物相关代谢特征与MASLD之间存在关联。代谢特征介导了PM2.5、PM10、二氧化氮和氮氧化物与MASLD之间的关联:结论:PM2.5、PM10、NO2和NOx相关代谢特征与MASLD之间可能存在关联,代谢特征介导了PM2.5、PM10、NO2和NOx对MASLD风险的增加:空气污染是一个重大的公共卫生问题,也是代谢功能障碍相关性脂肪性肝病(MASLD)的一个重要风险因素,然而,空气污染影响MASLD的机制仍不清楚。我们的研究利用大规模队列研究中 251 种代谢物的血清代谢综合数据,证明了代谢特征在空气污染导致的代谢性脂肪肝风险升高中起着至关重要的作用。这些结果与患者和政策制定者息息相关,因为它们表明与空气污染相关的代谢特征不仅可能与MASLD有关,而且还参与了介导PM2.5、PM10、二氧化氮和氮氧化物增加MASLD风险的过程。关注与空气污染相关的代谢特征的变化可能会为预防空气污染诱发的 MASLD 提供一个新的视角,并成为应对这一新兴公共卫生挑战的保护措施。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hepatology
医学-胃肠肝病学
CiteScore
46.10
自引率
4.30%
发文量
2325
审稿时长
30 days
期刊介绍:
The Journal of Hepatology is the official publication of the European Association for the Study of the Liver (EASL). It is dedicated to presenting clinical and basic research in the field of hepatology through original papers, reviews, case reports, and letters to the Editor. The Journal is published in English and may consider supplements that pass an editorial review.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


