Ultrasound-compatible 3D-printed Franz diffusion system for sonophoresis with microbubbles
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Sonophoresis is a topical drug delivery approach that utilises ultrasound as a physical stimulus to enhance permeation of active pharmaceutical ingredients through the skin. Only limited research has however been conducted to evaluate the potential of ultrasound-responsive drug carriers, such as gas microbubbles, in sonophoresis. Franz diffusion cells have been extensively used for measuring drug permeation in vitro; however, traditional systems lack compatibility with ultrasound and only limited characterisation of their acoustical behaviour has been carried out in previous research. To overcome this limitation, we designed and manufactured a novel Franz cell donor compartment coupled with a conventional glass receptor, and performed a functional characterisation of the assembly for application in sonophoresis with ultrasound-responsive agents (specifically imiquimod-loaded gas microbubbles). The donor was fabricated using a photoreactive resin via 3D printing and was designed to enable integration with a therapeutically relevant ultrasound source. The assembly was capable of effectively retaining liquids during prolonged incubation and the absorption of imiquimod onto the 3D-printed material was comparable to the one of glass. Moreover, a predictable ultrasound field could be generated at a target surface without any significant spatial distortion. Finally, we demonstrated applicability of the developed assembly in sonophoresis experiments with StratM®, wherein ultrasound stimulation in the presence of microbubbles resulted in significantly enhanced drug permeation through and partitioning within the membrane (2.96 ± 0.25 μg and 3.84 ± 0.39 μg) compared to passive diffusion alone (1.74 ± 0.29 μg and 2.29 ± 0.32 μg), over 24 h.
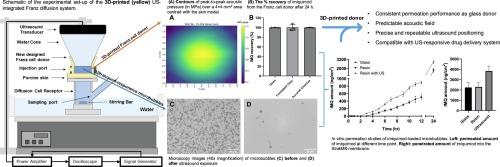
与超声波兼容的三维打印弗朗兹扩散系统,用于微气泡声波渗透。
声波透析是一种局部给药方法,它利用超声波作为物理刺激,增强活性药物成分在皮肤中的渗透。然而,目前只有有限的研究对超声响应药物载体(如气体微泡)在声波渗透中的潜力进行了评估。弗朗兹扩散细胞已被广泛用于体外药物渗透测量;然而,传统系统缺乏与超声波的兼容性,以往的研究仅对其声学行为进行了有限的表征。为了克服这一局限性,我们设计并制造了一种新型弗兰茨细胞供体室与传统的玻璃受体相结合,并对该组件进行了功能表征,以便将其应用于超声响应剂(特别是咪喹莫特载气微泡)的声波渗透。供体是使用光敏树脂通过三维打印制造的,其设计目的是与治疗相关的超声源集成。在长时间的培养过程中,该组件能够有效地保留液体,3D打印材料对咪喹莫特的吸收率与玻璃相当。此外,还能在目标表面产生可预测的超声场,而不会出现任何明显的空间扭曲。最后,我们在使用 StratM® 进行的声波透射实验中证明了所开发组件的适用性,与单纯的被动扩散(1.74 ± 0.29 μg 和 2.29 ± 0.32 μg)相比,在有微气泡存在的情况下,超声波刺激可在 24 小时内显著提高药物在膜内的渗透和分配(2.96 ± 0.25 μg 和 3.84 ± 0.39 μg)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


