Aggregation of therapeutic monoclonal antibodies due to thermal and air/liquid interfacial agitation stress: Occurrence, stability assessment strategies, aggregation mechanism, influencing factors, and ways to enhance stability
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
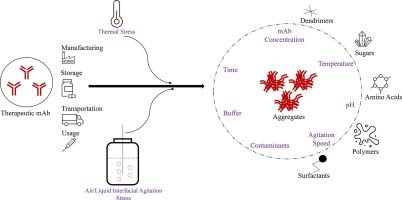
Therapeutic proteins, such as monoclonal antibodies (mAbs) are known to undergo stability related issues during various stages of product life cycle resulting in the formation of aggregates and fragments. Aggregates of mAb might result in reduced therapeutic activity and could cause various adverse immunogenic responses. Sample containing mAb undergo aggregation due to various types of stress factors, and there is always a continuous interest among researchers and manufacturers to determine the effect of different factors on the stability of mAb. Thermal stress and air/liquid interfacial agitation stress are among two of the common stress factors to which samples containing mAb are exposed to during various stages. Initial part of this review articles aims to provide a generalized understanding of aggregation of mAb such as size ranges of aggregates, aggregate types, stress factors, analytical techniques, permissible aggregate limits, and stability assessment methods. This article further aims to explain different aspects associated with aggregation of mAb in liquid samples due to thermal and air/liquid interfacial agitation stress. Under each stress category, the occurrence of stress during product life cycle, type of aggregates formed, mechanism of aggregation, strategies used by various researchers to expose mAb containing samples to stress, different factors affecting aggregation, fate of aggregates in human body fluids, and strategies used to enhance mAb stability has been explained in detail. The authors hope that this article provides a detailed understanding about stability of mAb due to thermal and air/liquid interfacial stress with relevance to product life cycle from manufacturing to administration into patients.
热应力和空气/液体界面搅拌应力导致治疗用单克隆抗体聚集:发生率、稳定性评估策略、聚集机制、影响因素以及提高稳定性的方法。
众所周知,单克隆抗体(mAbs)等治疗蛋白质在产品生命周期的各个阶段都会出现稳定性问题,从而形成聚集体和片段。mAb 的聚集体可能会导致治疗活性降低,并引起各种不良的免疫原性反应。含有 mAb 的样品会因各种应力因素而发生聚集,因此研究人员和制造商一直都在关注确定不同因素对 mAb 稳定性的影响。热应力和空气/液体界面搅拌应力是含有 mAb 的样品在不同阶段所面临的两种常见应力因素。本综述文章的第一部分旨在提供对 mAb 聚集的一般理解,如聚集体的尺寸范围、聚集体类型、应力因素、分析技术、允许的聚集体限值和稳定性评估方法。本文旨在进一步解释由于热应力和空气/液体界面搅拌应力而导致的液体样品中 mAb 聚集的不同方面。在每个应力类别下,都详细解释了产品生命周期中发生的应力、形成的聚集体类型、聚集机制、不同研究人员将含 mAb 样品暴露于应力的策略、影响聚集的不同因素、聚集体在人体体液中的去向以及用于提高 mAb 稳定性的策略。作者希望这篇文章能让人们详细了解热应力和空气/液体界面应力导致的 mAb 稳定性,这与产品从生产到给患者用药的生命周期息息相关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


