Temperature mapping of milling by dual centrifugation: A systematic investigation
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
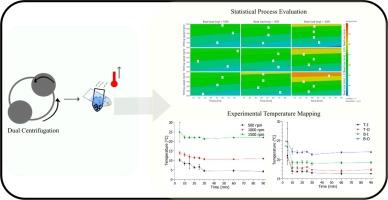
Using low quantities of drug compounds is often favorable in the early stages of drug development, especially for what require a large screening investigation to define the final formulation composition, such as nano- and microsuspensions. For that reason, the dual centrifugation approach has in the recent years been used due to its reproducible and fast-milling capacity with 40 samples in 2 mL vials simultaneously without the addition of cooling breaks due to a built-in cooling system. Nonetheless, heat can be dissipated into the samples during high-intensity milling, resulting in increased sample temperatures that potentially can affect thermolabile compounds and potential influence the obtained suspensions in the screening experiments if the used stabilizer has temperature dependent variations in the performance. Hence, a systematic investigation of the influence of different process parameters on the heat dissipation in samples during milling by the dual centrifugation approach was performed in the present study. It was found that the milling speed had the highest impact on the final sample temperature, but also other parameters, such as the bead loading, bead size, and placement in the centrifuge during milling had significantly influenced the final mean temperature of the milling media. Higher temperatures were obtained with higher bead loadings, i.e., 3000 mg milling beads/mL and milling speeds (1500 rpm), and when smaller milling beads, i.e., 0.1 mm, were used during production. The study further showed that higher temperatures were measured for samples located on the bottom disk during milling, and also when located on the outer placement on the sample disk. Upscale investigations showed immensely increased sample temperatures (almost up to boiling point) when samples were prepared under similar formulation parameters and milling speed as small-volume vials. Furthermore, the study indicated that the addition of drug compounds during suspension preparation decreased the final sample temperature compared to samples that only contained purified water due to energy absorption of the drug compound.
双离心研磨的温度分布:系统调查。
在药物开发的早期阶段,使用低剂量的药物化合物通常是有利的,特别是对于需要进行大量筛选调查以确定最终配方组成的药物,如纳米和微悬浮剂。因此,近年来,双离心方法因其可重复性和快速研磨能力而被广泛使用,通过内置的冷却系统,可同时将 40 个样品放入 2 mL 容量瓶中,而无需增加冷却时间。然而,在高强度研磨过程中,热量会散失到样品中,导致样品温度升高,如果使用的稳定剂性能随温度变化,则可能会影响热敏性化合物,并对筛选实验中获得的悬浮液产生潜在影响。因此,本研究采用双离心方法对研磨过程中不同工艺参数对样品散热的影响进行了系统研究。研究发现,研磨速度对最终样品温度的影响最大,但其他参数,如研磨珠装载量、研磨珠尺寸和研磨过程中在离心机中的放置位置,也对研磨介质的最终平均温度有显著影响。较高的研磨珠装载量(即 3000 毫克研磨珠/毫升)和研磨速度(1500 转/分钟),以及在生产过程中使用较小的研磨珠(即 0.1 毫米)时,可获得较高的温度。研究进一步表明,在研磨过程中,位于底部圆盘上的样品以及位于样品圆盘外侧的样品测得的温度更高。升级调查显示,在与小容量瓶相似的配方参数和研磨速度下制备样品时,样品温度大幅升高(几乎达到沸点)。此外,研究还表明,与只含有纯净水的样品相比,在悬浮液制备过程中添加药物化合物会降低最终样品的温度,这是因为药物化合物吸收了能量。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


