Population pharmacokinetic analysis and dosing optimization of colistin sulphate in lung transplant recipients with pneumonia: A prospective study
IF 4.9
2区 医学
Q1 INFECTIOUS DISEASES
International Journal of Antimicrobial Agents
Pub Date : 2024-09-26
DOI:10.1016/j.ijantimicag.2024.107346
引用次数: 0
Abstract
Objective
Currently, there is a lack of information on the clinical pharmacokinetics (PK), effectiveness, and safety of colistin sulphate (CS) in lung transplant recipients. This study aims to improve CS dosing regimens and evaluate its population PK in lung transplant recipients.
Methods
This study evaluated the clinical efficacy, microbiological efficacy, and adverse events of CS in lung transplant recipients. The NONMEM program was employed to construct the population PK model, and Monte Carlo simulations were executed to establish dosing regimens according to the probability of target attainment (PTA).
Results
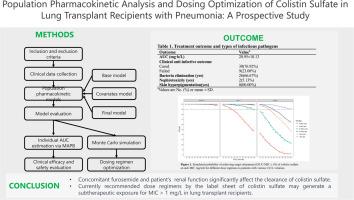
The study included 146 CS concentrations, spanning from 0.05 to 4.18 mg/L from 39 lung transplant recipients with multidrug-resistant Gram-negative bacteria. 26 (66.67%) patients successfully eradicated bacteria, and 30 (76.92%) patients had clinical cure or improvement. Additionally, only 2 (5.13%) patients developed CS-related nephrotoxicity. The PK profile was effectively represented by a one-compartmental model with linear elimination. Creatinine clearance and concomitant furosemide use were recognized as covariates influencing the clearance of CS. Based on the PTA results, a daily dosage of 1.5 million IU, divided into 2–3 administrations, could attain a PTA exceeding 90% for MIC ≤ 1 µg/mL at creatinine clearance of about 110 mL/min. However, this regimen would lead to insufficient exposure for MIC ≥ 2 µg/mL.
Conclusions
The clearance of CS is significantly influenced by concomitant furosemide use and renal function. The currently recommended dosing regimens by label sheet may result in subtherapeutic exposure for MIC exceeding 1 mg/L in lung transplant recipients.
硫酸可乐定在肺移植受者肺炎患者中的群体药代动力学分析和剂量优化:前瞻性研究。
目的:目前,缺乏有关硫酸可乐定在肺移植受者中的临床药代动力学(PK)、有效性和安全性的信息。本研究旨在改进硫酸可乐定的给药方案,并评估其在肺移植受者中的群体药代动力学(PopPK):本研究评估了硫酸可乐定在肺移植受者中的临床疗效、微生物学疗效和不良反应。采用 NONMEM 程序构建 PopPK 模型,并进行蒙特卡罗模拟,根据达到目标的概率(PTA)确定给药方案:研究共纳入了 39 名患有多重耐药革兰氏阴性菌的肺移植受者的 146 个硫酸可乐定浓度,范围从 0.05 到 4.18 mg/L。26例(66.67%)患者成功根除了细菌,30例(76.92%)患者临床治愈或好转。此外,只有 2 例(5.13%)患者出现了与硫酸可乐定相关的肾毒性。该药物的 PK 曲线通过线性消除的单室模型得到了有效体现。肌酐清除率(CrCL)和同时使用呋塞米被认为是影响硫酸可乐定清除率的协变量。根据 PTA 结果,每日剂量为 150 万 IU,分 2-3 次给药,当 CrCL 约为 110 mL/min 时,MIC ≤ 1 µg/mL 的 PTA 可超过 90%。然而,如果 MIC ≥ 2 µg/mL,该方案会导致暴露不足:结论:硫酸可乐定的清除率受同时使用呋塞米和肾功能的显著影响。目前标签页推荐的给药方案可能会导致肺移植受者在 MIC 超过 1 mg/L 时出现治疗暴露不足。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
21.60
自引率
0.90%
发文量
176
审稿时长
36 days
期刊介绍:
The International Journal of Antimicrobial Agents is a peer-reviewed publication offering comprehensive and current reference information on the physical, pharmacological, in vitro, and clinical properties of individual antimicrobial agents, covering antiviral, antiparasitic, antibacterial, and antifungal agents. The journal not only communicates new trends and developments through authoritative review articles but also addresses the critical issue of antimicrobial resistance, both in hospital and community settings. Published content includes solicited reviews by leading experts and high-quality original research papers in the specified fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


