Lyso-phosphatidylcholine as an interfacial stabilizer for parenteral monoclonal antibody formulations
IF 4.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2024-09-26
DOI:10.1016/j.ejpb.2024.114514
引用次数: 0
Abstract
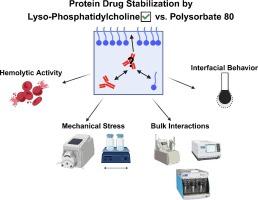
Therapeutic proteins suffer from physical and chemical instability in aqueous solution. Polysorbates and poloxamers are often added for protection against interfacial stress to prevent protein aggregation and particle formation. Previous studies have revealed that the hydrolysis and oxidation of polysorbates in parenteral formulations can lead to the formation of free fatty acid particles, insufficient long-term stabilization, and protein oxidation. Poloxamers, on the other hand, are considered to be less effective against protein aggregation. Here we investigated two lyso-phosphatidylcholines (LPCs) as potential alternative surfactants for protein formulations, focusing on their physicochemical behavior and their ability to protect against the formation of monoclonal antibody particles during mechanical stress.
The hemolytic activity of LPC was tested in varying ratios of plasma and buffer mixtures. LPC effectively stabilized mAb formulations when shaken at concentrations several orders of magnitude below the onset of hemolysis, indicating that the potential for erythrocyte damage by LPC is non-critical. LPC formulations subjected to mechanical stress through peristaltic pumping exhibited comparable protein particle formation to those containing polysorbate 80 or poloxamer 188. Profile analysis tensiometry and dilatational rheology indicated that the stabilizing effect likely arises from the formation of a viscoelastic film at approximately the CMC. Data gathered from concentration-gradient multi-angle light scattering and isothermal titration calorimetry support this finding. Surfactant desorption was evaluated through sub-phase exchange experiments. While LPCs readily desorbed from the interface, resorption occurred rapidly enough in the bulk solution to prevent protein adsorption. Overall, LPCs behave similarly to polysorbate with respect to interfacial stabilization and show promise as a potential substitute for polysorbate in parenteral protein formulations.
溶血磷脂酰胆碱作为肠外单克隆抗体制剂的界面稳定剂。
治疗用蛋白质在水溶液中存在物理和化学不稳定性。为了防止蛋白质聚集和颗粒形成,通常会添加聚山梨醇酯和聚氧乙烯酰胺来抵御界面应力。以往的研究表明,聚山梨醇酯在肠外制剂中的水解和氧化会导致游离脂肪酸颗粒的形成、长期稳定性不足以及蛋白质氧化。另一方面,聚氧乙烯酰胺被认为对防止蛋白质聚集的效果较差。在此,我们研究了两种溶血磷脂酰胆碱(LPC),将其作为蛋白质配方的潜在替代表面活性剂,重点关注它们的物理化学行为以及在机械应力作用下防止单克隆抗体颗粒形成的能力。在不同比例的血浆和缓冲液混合物中测试了 LPC 的溶血活性。当摇动浓度低于溶血起始浓度几个数量级时,LPC 可有效稳定 mAb 制剂,这表明 LPC 对红细胞的潜在破坏并不严重。通过蠕动泵施加机械应力的 LPC 制剂与含有聚山梨醇酯 80 或 poloxamer 188 的制剂相比,蛋白质颗粒的形成情况相当。剖面分析张力测定法和扩张流变学表明,稳定效果可能来自于在大约 CMC 处形成的粘弹性薄膜。浓度梯度多角度光散射和等温滴定量热法收集的数据也支持这一结论。通过亚相交换实验对表面活性剂解吸进行了评估。虽然 LPC 很容易从界面上解吸,但在大体积溶液中的再吸附速度很快,足以阻止蛋白质的吸附。总之,LPC 在界面稳定方面的表现与聚山梨醇酯类似,有望成为肠外蛋白质制剂中聚山梨醇酯的潜在替代品。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


