Stability evaluation from hydrogen peroxide spiking studies
IF 4.3
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
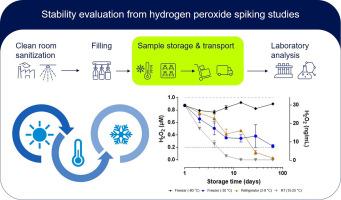
The ability to detect traces of hydrogen peroxide (H2O2) in water is an important prerequisite to ensure a safe and reliable use of H2O2 for isolator or cleanroom sanitization. While the residual airborne H2O2 concentration can be easily monitored, detection of trace H2O2 residues in aseptically filled drug products is challenging. In an industrial setting, samples must be pulled, handled, stored, and transported before analysis takes place. Therefore, knowledge about the analyte stability in the relevant matrix is crucial to ensure correct results.
The objective of this study was to provide stability data for the analyte at low concentrations and in aqueous solutions. For this, H2O2 was spiked into four different aqueous matrices at two different concentrations and stored up to 60 days at four different storage temperatures. The tested matrices included water, buffer, and an exemplary excipient solution with and without additional protein.
A developmental quantitative, fluorometric Amplex UltraRed assay was applied for analysis. The results show clearly, that of the four storage temperatures investigated, only -80 °C resulted in reasonably good recovery of the spiked H2O2 content within two weeks or even up to two months of storage.
从过氧化氢加标研究中评估稳定性。
检测水中痕量过氧化氢(H2O2)的能力是确保安全可靠地使用 H2O2 进行隔离器或洁净室消毒的重要先决条件。虽然空气中残留的 H2O2 浓度很容易监测,但无菌灌装药品中痕量 H2O2 残留的检测却很困难。在工业环境中,在进行分析之前必须提取、处理、储存和运输样品。因此,了解分析物在相关基质中的稳定性对于确保结果正确至关重要。本研究的目的是提供分析物在低浓度水溶液中的稳定性数据。为此,在四种不同的水溶液基质中添加了两种不同浓度的 H2O2,并在四种不同的储存温度下储存长达 60 天。测试的基质包括水、缓冲液和含或不含额外蛋白质的示例溶液。分析中使用了发展定量荧光测定法 Amplex UltraRed。结果清楚地表明,在调查的四种储存温度中,只有 -80°C 能在两周甚至长达两个月的储存时间内使加标 H2O2 的含量得到相当好的恢复。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


