Evaluation and application of population pharmacokinetic models for optimising linezolid treatment in non-adherence multidrug-resistant tuberculosis patients
IF 4.3
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Background
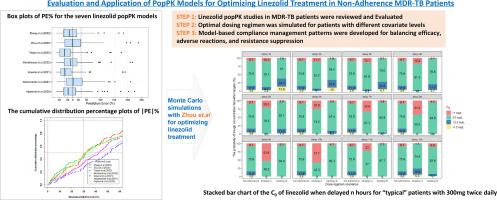
Population pharmacokinetic (popPK) models can optimise linezolid dosage regimens in patients with multidrug-resistant tuberculosis (MDR-TB); however, unknown cross-centre precision and poor adherence remain problematic. This study aimed to assess the predictive ability of published models and use the most suitable model to optimise dosage regimens and manage compliance.
Methods
One hundred fifty-eight linezolid plasma concentrations from 27 patients with MDR-TB were used to assess the predictive performance of published models. Prediction-based metrics and simulation-based visual predictive checks were conducted to evaluate predictive ability. Individualised remedial dosing regimens for various delayed scenarios were optimised using the most suitable model and Monte Carlo simulations. The influence of covariates, scheduled dosing intervals, and patient compliance were assessed.
Results
Seven popPK models were identified. Body weight and creatinine clearance were the most frequently identified covariates influencing linezolid clearance. The model with the best performance had a median prediction error (PE%) of -1.62 %, median absolute PE of 29.50 %, and percentages of PE within 20 % (F20, 36.97 %) and 30 % (F30, 51.26 %). Monte Carlo simulations indicated that a twice-daily 300 mg linezolid dose may be more efficient than 600 mg once daily. For the ‘typical’ patient treated with 300 mg twice daily, half the dosage should be taken after a delay of ≥ 3 h.
Conclusions
Monte Carlo simulations based on popPK models can propose remedial regimens for delayed doses of linezolid in patients with MDR-TB. Model-based compliance management patterns are useful for balancing efficacy, adverse reactions, and resistance suppression.
评估和应用群体药代动力学模型优化对不依从的耐多药结核病患者的利奈唑胺治疗
背景:群体药代动力学(popPK)模型可以优化耐多药结核病(MDR-TB)患者的利奈唑胺剂量方案;然而,跨中心精确性未知和依从性差仍是问题所在。本研究旨在评估已发表模型的预测能力,并使用最合适的模型来优化剂量方案和管理依从性:方法: 使用 27 名 MDR-TB 患者的 158 例利奈唑胺血浆浓度来评估已发表模型的预测性能。采用基于预测的指标和基于模拟的视觉预测检查来评估预测能力。利用最合适的模型和蒙特卡洛模拟优化了各种延迟情况下的个性化补救用药方案。评估了协变量、计划给药间隔和患者依从性的影响:结果:确定了七个 popPK 模型。体重和肌酐清除率是影响利奈唑胺清除率最常见的协变量。性能最好的模型的中位预测误差(PE%)为-1.62%,中位绝对预测误差为 29.50%,预测误差百分比在 20% (F20, 36.97%) 和 30% (F30, 51.26%) 以内。蒙特卡罗模拟显示,每天两次 300 毫克利奈唑胺的剂量可能比每天一次 600 毫克的剂量更有效。对于每日两次服用 300 毫克的 "典型 "患者,应在延迟≥ 3 小时后服用一半的剂量:基于 popPK 模型的蒙特卡罗模拟可为 MDR-TB 患者延迟服用利奈唑胺提出补救方案。基于模型的依从性管理模式有助于平衡疗效、不良反应和耐药性抑制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


