Zebrafish Foxl2l functions in proliferating germ cells for female meiotic entry
IF 2.5
3区 生物学
Q2 DEVELOPMENTAL BIOLOGY
引用次数: 0
Abstract
Zebrafish sex differentiation is a complicated process and the detailed mechanism has not been fully understood. Here we characterized a transcription factor, Foxl2l, which participates in female oogenesis. We show that it is expressed specifically in proliferating germ cells in juvenile gonads and mature ovaries. We have used CRISPR-Cas9 to generate zebrafish deficient in foxl2l expression. Zebrafish with foxl2l−/− are all males, and this female-to-male sex reversal cannot be reversed by tp53 mutation, indicating this sex reversal is unrelated to cell death. We have generated transgenic fish expressing GFP under the control of foxl2l promoter to track the development of foxl2l + -germ cells; these cells failed to enter meiosis and accumulated as cystic cells in the foxl2l−/− mutant. Our RNA-seq analysis also showed the reduced expression of genes in meiosis and oogenesis among other affected pathways. All together, we show that zebrafish Foxl2l is a nuclear factor controlling the expression of meiotic and oogenic genes, and its deficiency leads to defective meiotic entry and the accumulation of premeiotic germ cells.
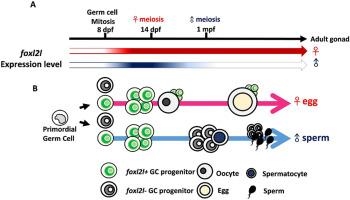
斑马鱼 Foxl2l 在增殖生殖细胞中发挥着雌性减数分裂入口的功能。
斑马鱼的性别分化是一个复杂的过程,其详细机制尚未完全清楚。在这里,我们对参与雌性卵子发生的转录因子 Foxl2l 进行了鉴定。我们发现它在幼年性腺和成熟卵巢的增殖生殖细胞中特异性表达。我们利用CRISPR-Cas9技术生成了缺乏Foxl2l表达的斑马鱼。foxl2l-/-斑马鱼均为雄性,这种雌性到雄性的性别逆转不能通过tp53突变逆转,表明这种性别逆转与细胞死亡无关。我们生成了在foxl2l启动子控制下表达GFP的转基因鱼,以追踪foxl2l+-胚芽细胞的发育过程;在foxl2l-/-突变体中,这些细胞未能进入减数分裂,而是以囊状细胞的形式积累。我们的RNA-seq分析还显示,减数分裂和卵子发生中的基因表达减少,其他受影响的途径也是如此。综上所述,我们发现斑马鱼Foxl2l是一种控制减数分裂和卵子生成基因表达的核因子,它的缺乏会导致减数分裂进入缺陷和减数分裂前生殖细胞的积累。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental biology
生物-发育生物学
CiteScore
5.30
自引率
3.70%
发文量
182
审稿时长
1.5 months
期刊介绍:
Developmental Biology (DB) publishes original research on mechanisms of development, differentiation, and growth in animals and plants at the molecular, cellular, genetic and evolutionary levels. Areas of particular emphasis include transcriptional control mechanisms, embryonic patterning, cell-cell interactions, growth factors and signal transduction, and regulatory hierarchies in developing plants and animals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


