Pax2a, Pax5 and Cdh1-β-catenin, but not Wnt, protect sensory hair cells from destabilizing effects of fgf signaling on cell adhesion
IF 2.5
3区 生物学
Q2 DEVELOPMENTAL BIOLOGY
引用次数: 0
Abstract
During inner ear development, specification of sensory epithelia requires dynamic regulation of Fgf signaling. In zebrafish, high levels of Fgf are necessary and sufficient to specify the utricular/vestibular macula, whereas the saccular/auditory macula requires a discreet lower level of Fgf. Transcription factors Pax2a and Pax5 act downstream of Fgf to help specify utricular identity, loss of which leads to sporadic extrusion of hair cells from the utricular macula. The mechanism for utricular instability is not clear but is potentially related to reduced expression of cdh1/Ecad caused by disruption of pax2a. Here we find that utricular hair cells in pax2−/− and pax5−/− mutants gradually lose adhesive contact with the macula, leading to ejection of intact hair cells from either the basal or apical surface. The phenotype is far more severe in pax2a−/− mutants and is progressive, resulting in loss of large swaths of the utricular hair cells by 82 hpf. Instability is caused by elevated Fgf signaling in the utricle, as modest reduction of Fgf signaling with a low dose of SU5402 prevents hair cell loss in pax2a−/− mutants. Misexpression of cdh1/Ecad in pax2a−/− mutants partially rescues pax2a−/− mutants. Elevating β-catenin levels by treatment with BIO, or misexpression of a mutant form of β-catenin lacking transcriptional activity but retaining cell adhesion function, fully rescues pax2a−/− mutants. In contrast, Wnt signaling is not required for utricular stability. Thus, Pax2/5 factors serve to counteract the destabilizing effects of elevated Fgf signaling needed to specify utricular identity.
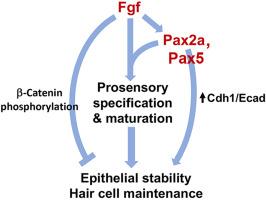
Pax2a、Pax5和Cdh1-β-catenin(而非Wnt)能保护感觉毛细胞免受Fgf信号对细胞粘附的不稳定影响。
在内耳发育过程中,感觉上皮的特定需要 Fgf 信号的动态调控。在斑马鱼中,高水平的 Fgf 是明确胞质/前庭黄斑的必要且充分条件,而囊质/听觉黄斑则需要低水平的 Fgf。转录因子 Pax2a 和 Pax5 在 Fgf 的下游发挥作用,帮助确定胞丘的特征,而转录因子 Pax2a 和 Pax5 的缺失会导致零星的毛细胞从胞丘挤出。胞毛不稳定的机制尚不清楚,但可能与 pax2a 的破坏导致 cdh1/Ecad 表达减少有关。在这里,我们发现 pax2-/-和 pax5-/-突变体的胞室毛细胞会逐渐失去与黄斑的粘附接触,导致完整的毛细胞从基底或顶端表面脱落。pax2a-/- 突变体的表型要严重得多,而且是渐进性的,到 82 hpf 时会导致大片胞毛细胞丧失。不稳定性是由子宫中升高的Fgf信号引起的,因为用低剂量的SU5402适度减少Fgf信号可防止pax2a-/-突变体中毛细胞的丧失。在pax2a-/-突变体中错误表达cdh1/Ecad可部分挽救pax2a-/-突变体。通过 BIO 处理提高β-catenin 水平,或错误表达缺乏转录活性但保留细胞粘附功能的突变形式的β-catenin,可完全拯救 pax2a-/- 突变体。与此相反,胞室稳定性并不需要 Wnt 信号传导。因此,Pax2/5因子的作用是抵消Fgf信号升高所产生的不稳定影响,而Fgf信号升高是指定胞室特性所必需的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental biology
生物-发育生物学
CiteScore
5.30
自引率
3.70%
发文量
182
审稿时长
1.5 months
期刊介绍:
Developmental Biology (DB) publishes original research on mechanisms of development, differentiation, and growth in animals and plants at the molecular, cellular, genetic and evolutionary levels. Areas of particular emphasis include transcriptional control mechanisms, embryonic patterning, cell-cell interactions, growth factors and signal transduction, and regulatory hierarchies in developing plants and animals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


