Advent of oral medications for the treatment of hereditary angioedema
Abstract
Background
Hereditary angioedema (HAE) is a rare genetic disorder characterized by unpredictable, debilitating episodes of submucosal and/or subcutaneous tissue swelling, which may be life-threatening depending on anatomic location. The two primary management strategies for HAE are ready access to effective on-demand treatment in all patients and the prevention of attacks (short-term prophylaxis [STP] and long-term prophylaxis [LTP]) in appropriate patients. All approved on-demand and most LTP medications require subcutaneous or intravenous administration. Injection-related challenges include trypanophobia (fear of needles), difficulty with self-administration, injection-site reactions (e.g., pain, erythema, bleeding, bruising), and anxiety—all contributing to poor compliance and administration delays. Oral HAE treatments may improve outcomes by reducing treatment barriers.
Aim
To review oral therapies, approved or in development, for on-demand treatment and/or prevention of HAE attacks.
Materials and Methods
To provide a comprehensive review, data was obtained from publicly available resources through a targeted PubMed literature review and supplemented by information provided on company websites (search cutoff of May 31, 2024).
Results
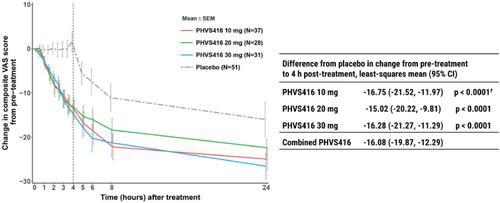
Berotralstat, an oral plasma kallikrein (PKa) inhibitor, is approved for LTP. Sebetralstat, another PKa inhibitor, is the investigational first oral on-demand HAE treatment to complete a phase 3 trial. Deucrictibant, an oral bradykinin B2 receptor antagonist, has completed phase 2 trials for on-demand therapy and LTP. Several other oral PKa inhibitors (ATN249, VE-4666, and VE-4062) are in early development for LTP.
Conclusion
Substantial advances have been made in the development of oral treatments for HAE. These treatments have the potential to improve and optimize clinical outcomes, satisfaction, and quality of life among patients with HAE.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


