Time Trends, Regional Variation and Associations of Low-Allergy Formula Prescribing in England
Abstract
Background
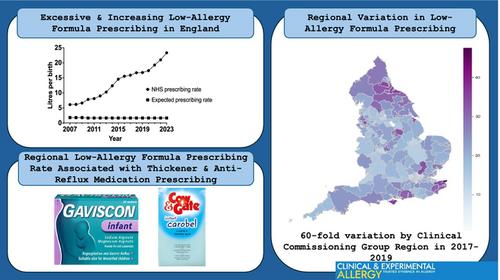
Cow's milk allergy (CMA) overdiagnosis appears to be increasing and is associated with excessive low-allergy formula prescription. We evaluated recent trends and regional variation in low-allergy formula prescribing for CMA in England, and assessed potential risk factors for higher prescribing rates.
Methods
Data on national and regional prescribing of low-allergy formulas were extracted from England's electronic prescription database using R. Region-level factors were evaluated for potential associations with regional low-allergy formula prescription rates using multivariate linear regression. Analysis of national prescribing trends covered 2007–2023, analysis of regional variation and region-level factors examined 2017–2019, prior to a re-organisation of the regional healthcare structure in England.
Results
Low-allergy formula prescribing increased from 6.1 to 23.3 L per birth nationally, between 2007 and 2023. Regional prescribing rate varied from 0.8 to 47.6 L per birth in 2017–2019. We found significant associations between regional low-allergy formula prescribing rate and regional prescribing rates for milk feed thickeners Gaviscon Infant and Carobel Instant (β = 0.10, p < 0.01), and for other anti-reflux medications used in young children (β = 0.89 p < 0.01). Inconsistent associations were seen with prescribing junior adrenaline auto-injectors and oral antibiotics. A model including these four variables accounted for 37% of regional variation in low-allergy formula prescribing rate. Region-level socio-economic deprivation, CMA guideline recommendations and paediatric allergy service provision were not associated with low-allergy formula prescribing.
Conclusions
Low-allergy formula prescribing in England is increasing, varies significantly by region and is consistently associated with prescribing rates for milk feed thickeners and other anti-reflux medication for young children. Community prescribing behaviours may be important determinants of CMA overdiagnosis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


