Synthesis of trisaccharide antigens featuring colitose, abequose and fucose residues and assessment of antibody binding on antigen arrays
IF 2.4
3区 化学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Deoxy-hexose sugars, such as rhamnose and quinovose, and the dideoxy-hexoses colitose, abequose, and tyvelose are highly antigenic given that they are absent from animal glycoconjugates. To investigate the specificity of antibodies towards structurally similar carbohydrate epitopes found in bacteria, we synthesized trisaccharides containing colitose, abequose, and fucose motifs. Each trisaccharide was designed with a spacer ending with a primary amino group. These trisaccharide constructs were immobilized on O-succinimide coated glass slides alongside bacterial lipopolysaccharides (LPS) containing colitose, abequose, and fucose residues. We compared the recognition of the synthetic trisaccharides and natural LPS including structurally related epitopes by monoclonal and polyclonal antibodies targeting bacterial LPS. Additionally, we used arrays displaying the synthetic trisaccharides and natural LPS to assess the variability of IgA reactivity from breast milk samples towards the carbohydrate antigens. The results obtained underlined the cross-reactivity of polyclonal antibodies towards structurally related carbohydrate antigens and revealed a broad reactivity of breast milk-derived IgA towards the carbohydrate antigens tested. The significant cross-reactivity of antibodies towards structurally related LPS antigens may lead to false-positive detection of bacterial serotypes when used for diagnostic purposes.
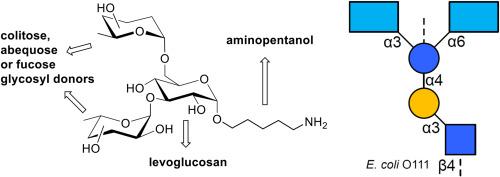
合成以可乐糖、阿贝糖和岩藻糖残基为特征的三糖抗原,并评估抗原阵列上的抗体结合情况。
鼠李糖和喹诺酮糖等脱氧己糖以及可乐糖、阿贝糖和岩藻糖等双脱氧己糖具有很强的抗原性,因为动物糖结合物中没有这些糖。为了研究抗体对细菌中结构相似的碳水化合物表位的特异性,我们合成了含有可乐糖、阿贝果糖和岩藻糖基团的三糖。每种三糖都设计了一个以伯氨基结尾的间隔。将这些三糖构建体固定在涂有 O-琥珀酰亚胺的玻璃载玻片上,同时固定的还有含有可立糖、阿贝库糖和岩藻糖残基的细菌脂多糖(LPS)。我们比较了针对细菌 LPS 的单克隆和多克隆抗体对合成三糖和天然 LPS(包括结构相关的表位)的识别率。此外,我们还使用显示合成三糖和天然 LPS 的阵列来评估母乳样本中 IgA 对碳水化合物抗原反应性的变异性。研究结果表明,多克隆抗体对结构相关的碳水化合物抗原具有交叉反应性,并显示母乳中的 IgA 对测试的碳水化合物抗原具有广泛的反应性。在用于诊断时,抗体对结构相关的 LPS 抗原的明显交叉反应可能会导致对细菌血清型的假阳性检测。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbohydrate Research
化学-生化与分子生物学
CiteScore
5.00
自引率
3.20%
发文量
183
审稿时长
3.6 weeks
期刊介绍:
Carbohydrate Research publishes reports of original research in the following areas of carbohydrate science: action of enzymes, analytical chemistry, biochemistry (biosynthesis, degradation, structural and functional biochemistry, conformation, molecular recognition, enzyme mechanisms, carbohydrate-processing enzymes, including glycosidases and glycosyltransferases), chemical synthesis, isolation of natural products, physicochemical studies, reactions and their mechanisms, the study of structures and stereochemistry, and technological aspects.
Papers on polysaccharides should have a "molecular" component; that is a paper on new or modified polysaccharides should include structural information and characterization in addition to the usual studies of rheological properties and the like. A paper on a new, naturally occurring polysaccharide should include structural information, defining monosaccharide components and linkage sequence.
Papers devoted wholly or partly to X-ray crystallographic studies, or to computational aspects (molecular mechanics or molecular orbital calculations, simulations via molecular dynamics), will be considered if they meet certain criteria. For computational papers the requirements are that the methods used be specified in sufficient detail to permit replication of the results, and that the conclusions be shown to have relevance to experimental observations - the authors'' own data or data from the literature. Specific directions for the presentation of X-ray data are given below under Results and "discussion".
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


