Molecular profiling of a rat model of vascular dementia: Evidences from proteomics, metabolomics and experimental validations
IF 2.7
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
Abstract
Decrease of cerebral blood flow is the primary cause of vascular dementia (VD), but its pathophysiological mechanisms are still not known. This study aims to profile the molecular changes of a rat model of VD induced by bilateral common carotid artery ligation. The Morris water maze and new object recognition tasks were used to test the cognitive function of rats. Hematoxylin and Eosin (HE) staining was used to detect pathological changes in the hippocampus. After confirming the model, proteomics was used to detect differentially expressed proteins in the hippocampus, and metabolomics was used to detect differential metabolites in rat serum. Thereafter, bioinformatics were used to integrate and analyze the potential molecular profile. The results showed that compared with the sham control group, the spatial and recognition memory of the rats were significantly reduced, and pathological changes were observed in the hippocampal CA1 region of the model group. Proteomic analysis suggested 206 differentially expressed proteins in the hippocampus of VD rats, with 117 proteins upregulated and 89 downregulated. Protein-protein interaction network analysis suggested that those differentially expressed proteins might play crucial roles in lipid metabolism, cell adhesion, intracellular transport, and signal transduction. Metabolomics analysis identified 103 differential metabolites, and comparison with the human metabolome database revealed 22 common metabolites, which predicted 265 potential targets. Afterwards, by intersecting the predicted results from metabolomics with the differentially expressed proteins from proteomics, we identified five potential targets, namely ACE, GABBR1, Rock1, Abcc1 and Mapk10. Furthermore, western blotting confirmed that compared with control group, hippocampal GABBR1 and Rock1 were enhanced in the model group. Together, this study showed the molecular profile of VD rats through a combination of proteomics, metabolomics, and experimental confirmation methods, offering crucial molecular targets for the diagnosis and treatment of VD.
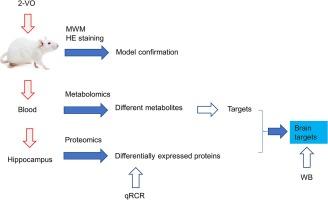
大鼠血管性痴呆模型的分子剖析:蛋白质组学、代谢组学和实验验证的证据。
脑血流减少是血管性痴呆(VD)的主要病因,但其病理生理机制仍不清楚。本研究旨在剖析双侧颈总动脉结扎诱导的血管性痴呆大鼠模型的分子变化。研究采用 Morris 水迷宫和新物体识别任务测试大鼠的认知功能。血色素和伊红(HE)染色用于检测海马的病理变化。确认模型后,利用蛋白质组学检测海马中不同表达的蛋白质,并利用代谢组学检测大鼠血清中不同的代谢物。随后,生物信息学被用来整合和分析潜在的分子特征。结果显示,与假对照组相比,模型组大鼠的空间记忆和识别记忆明显减弱,海马CA1区出现病理变化。蛋白质组学分析表明,VD大鼠海马中有206个蛋白表达不同,其中117个蛋白上调,89个蛋白下调。蛋白质-蛋白质相互作用网络分析表明,这些差异表达的蛋白质可能在脂质代谢、细胞粘附、细胞内转运和信号转导中发挥关键作用。代谢组学分析发现了103种差异代谢物,与人类代谢组数据库比较发现了22种常见代谢物,预测出了265个潜在靶点。随后,通过将代谢组学的预测结果与蛋白质组学的差异表达蛋白进行交叉分析,我们确定了五个潜在靶点,分别是ACE、GABBR1、Rock1、Abcc1和Mapk10。此外,Western 印迹还证实,与对照组相比,模型组海马 GABBR1 和 Rock1 的表达增强。总之,本研究通过蛋白质组学、代谢组学和实验证实方法的结合,展示了VD大鼠的分子特征,为VD的诊断和治疗提供了重要的分子靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


