Remote ischemic conditioning slows blood-retinal barrier damage in type 1 diabetic rats
IF 2.7
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
Abstract
Diabetic retinopathy (DR) is one of the major complications of diabetes and can cause severe visual impairment. Blood-retina barrier (BRB) destruction resulted from chronic hyperglycemia underlines its major pathological process. However, current treatments have limited efficacy and may even cause serious complications. Remote ischemic conditioning (RIC), through repeated transient mechanical occlusion of limb blood vessels, has been confirmed to promote blood–brain barrier integrity after stroke, but its role in BRB disruption has not been elucidated. This study aimed to investigate the protective effects of RIC on the BRB in diabetic rats and its potential mechanisms. 48 Sprague-Dawley rats were randomly assigned to the Sham group, Sham + RIC group, diabetes mellitus (DM) group and DM+RIC group. The diabetic model was successfully induced by intraperitoneal injection of streptozotocin. RIC treatment was administered daily and lasted for 9 weeks. In functional analysis, RIC improved the retinal function based on electroretinogram data and reduced the leakage of BRB in diabetic rats. In proteomic analysis, tight junction pathway was enriched after RIC treatment, in which Patj gene was significantly increased. We also found that RIC increased mRNA levels of Patj, claudin-1 and zonula occludens (ZO)-1, protein expression of claudin-1 when compared with diabetic models. In conclusion, RIC slowed BRB damage in diabetic rats, which may be related to the preservation of tight junction proteins. RIC may be a promising protective strategy for the treatment of DR.
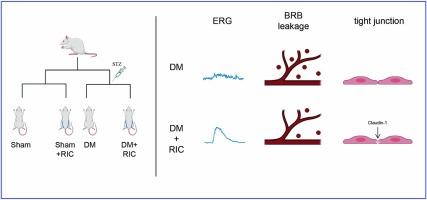
远程缺血调节可减缓 1 型糖尿病大鼠的血液-视网膜屏障损伤。
糖尿病视网膜病变(DR)是糖尿病的主要并发症之一,可导致严重的视力损害。长期高血糖导致的血-视网膜屏障(BRB)破坏是其主要病理过程。然而,目前的治疗方法疗效有限,甚至可能引起严重的并发症。远程缺血调理(RIC)通过反复短暂机械性闭塞肢体血管,已被证实能促进卒中后血脑屏障的完整性,但其在血脑屏障破坏中的作用尚未阐明。本研究旨在探讨 RIC 对糖尿病大鼠血脑屏障的保护作用及其潜在机制。48 只 Sprague-Dawley 大鼠被随机分为 Sham 组、Sham + RIC 组、糖尿病(DM)组和 DM+RIC 组。腹腔注射链脲佐菌素(STZ)成功诱导出糖尿病模型。RIC 治疗每天进行,持续 9 周。在功能分析中,根据视网膜电图数据,RIC 改善了糖尿病大鼠的视网膜功能,并减少了 BRB 的渗漏。在蛋白质组学分析中,RIC 治疗后紧密连接通路富集,其中 Patj 基因显著增加。我们还发现,与糖尿病模型相比,RIC 增加了 Patj、claudin-1 和 zonula occludens (ZO)-1 的 mRNA 水平,以及 claudin-1 的蛋白表达。总之,RIC 可减缓糖尿病大鼠 BRB 的损伤,这可能与保护紧密连接蛋白有关。RIC可能是治疗DR的一种很有前景的保护策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


