SecM leader peptide as an allosteric translation inhibitor: a molecular dynamics study
IF 2.8
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. General subjects
Pub Date : 2024-09-26
DOI:10.1016/j.bbagen.2024.130715
引用次数: 0
Abstract
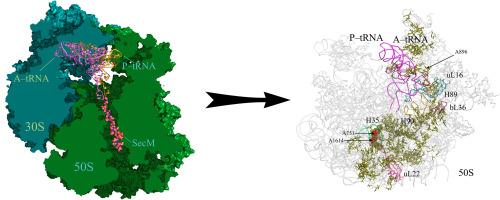
The SecM leader peptide regulates translation of the SecA protein, being a part of the Sec translocase, that reversibly arrests the ribosome. In the present study the structure of the SecM complex with the E. coli A/A,P/P–ribosome was obtained by means of docking and molecular dynamics simulation methods. It has been established that binding of the SecM leader peptide in the nascent peptide exit tunnel leads to a turn of the aminoacylating proline residue away from the C–terminal SecM glycine residue, which is adverse to the peptidyltransferase reaction. Besides, the SecM binding leads to a disturbance of the A–tRNA contacts with the tip of the H38 helix of the 23S rRNA (the A–site finger, ASF) and ribosomal protein uL16. Allosteric interrelation between these events has been proved by a construction of networks of concerted changes in non–covalent interactions throughout the whole ribosome, whereupon the A1614 and A751 residues of the 23S rRNA in the exit tunnel that formed stacking interactions with the SecM residues during the MD simulations, were found to be the principal triggers, inducing crucial alterations in the A–tRNA binding. The allosteric signal from the SecM peptide to the ASF, according to our model, is transmitted through ribosomal protein uL22, and there is reason to believe that this sensor is used not only by the SecM leader peptide, but also by other peptides that cause translation arrest.
作为异位翻译抑制剂的 SecM 领导肽:分子动力学研究
SecM 头肽调节 SecA 蛋白的翻译,SecA 蛋白是 Sec 易位酶的一部分,可逆性地抑制核糖体。本研究通过对接和分子动力学模拟方法,获得了 SecM 与大肠杆菌 A/A,P/P-核糖体的复合物结构。研究证实,SecM 头肽在新生肽出口隧道中的结合会导致氨基酰化脯氨酸残基转向远离 C 端 SecM 甘氨酸残基,从而不利于肽基转移酶反应。此外,SecM 的结合导致 A-tRNA 与 23S rRNA 的 H38 螺旋顶端(A 位点指,ASF)和核糖体蛋白 uL16 的接触受到干扰。通过构建整个核糖体非共价相互作用的协同变化网络,证明了这些事件之间的异化作用相互关系。在 MD 模拟过程中,23S rRNA 出口隧道中与 SecM 残基形成堆叠相互作用的 A1614 和 A751 残基被发现是主要的触发器,诱发了 A-tRNA 结合的关键变化。根据我们的模型,从 SecM 肽到 ASF 的异构信号是通过核糖体蛋白 uL22 传递的,因此有理由相信,不仅 SecM 头肽,而且其他导致翻译停止的肽也会使用这种传感器。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochimica et biophysica acta. General subjects
生物-生化与分子生物学
CiteScore
6.40
自引率
0.00%
发文量
139
审稿时长
30 days
期刊介绍:
BBA General Subjects accepts for submission either original, hypothesis-driven studies or reviews covering subjects in biochemistry and biophysics that are considered to have general interest for a wide audience. Manuscripts with interdisciplinary approaches are especially encouraged.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


