Establishment of the Meyer-Overton correlation in an artificial membrane without protein
IF 2.8
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. General subjects
Pub Date : 2024-09-27
DOI:10.1016/j.bbagen.2024.130717
引用次数: 0
Abstract
Background
The potency of anesthetics with various structures increases exponentially with lipophilicity, which is the Meyer-Overton (MO) correlation discovered over 120 years ago. The MO correlation was also observed with various biological effects and chemicals, including alcohols; thus, the correlation represents a fundamental relationship between chemicals and organisms. The MO correlation was explained by the lipid and protein theories, although the principle remains unknown because these are still debating.
Methods
The gentle hydration method was used to form giant unilamellar vesicles (GUVs) consisting of high- and low-melting phospholipids and cholesterol in the presence of n-alcohols (C2-C12). Confocal fluorescence microscopy was used to determine the percentage of GUVs with domains in relation to the n-alcohol concentrations.
Results
n-Alcohols inhibited the domain formation of GUVs, and the half inhibitory concentration (IC50) in the aqueous phase (Cw) decreased exponentially with increasing chain length (lipophilicity). In contrast, the membrane concentrations (Cm) of alcohols for the inhibition, which is a product of the membrane-water partition coefficient and the IC50 values, remained constant irrespective of the chain length.
Conclusions
The MO correlation is established in GUVs, which supports the lipid theory. When alcohols reach the same critical concentration in the membrane, similar biological effects appear irrespective of the chain length, which is the principle underlying the MO correlation.
General significance
The protein theory states that a highly lipophilic compound targets minor membrane proteins due to the low Cw. However, our lipid theory states that the compound targets various membrane proteins due to the high Cm.
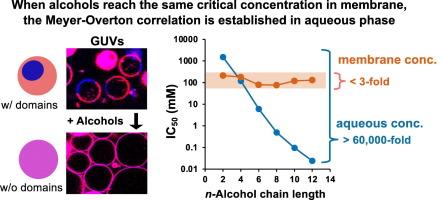
在不含蛋白质的人工膜中建立迈耶-奥弗顿相关性。
背景:各种结构的麻醉剂的药效随着亲脂性的增加而呈指数增长,这就是 120 多年前发现的迈耶-奥弗顿(MO)相关性。在各种生物效应和化学品(包括酒精)中也观察到了 MO 相关性;因此,这种相关性代表了化学品和生物体之间的一种基本关系。MO 相关性可以用脂质和蛋白质理论来解释,但其原理仍然不明,因为这些理论仍在争论之中:方法:在正丙醇(C2-C12)存在的情况下,使用温和水合法形成由高熔点和低熔点磷脂和胆固醇组成的巨型单拉美拉尔囊泡 (GUV)。结果:正丁醇抑制了 GUV 的结构域形成,水相(Cw)中的半抑制浓度(IC50)随着链长(亲脂性)的增加呈指数下降。与此相反,抑制作用的醇膜浓度(Cm),即膜-水分配系数与 IC50 值的乘积,无论链长多少都保持不变:结论:在 GUV 中建立了 MO 相关性,这支持了脂质理论。当醇在膜中达到相同的临界浓度时,无论链长如何,都会出现相似的生物效应,这就是 MO 相关性的基本原理:一般意义:蛋白质理论认为,高亲脂性化合物由于Cw较低,会针对次要膜蛋白。然而,我们的脂质理论则认为,由于高 Cm,化合物会针对各种膜蛋白。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochimica et biophysica acta. General subjects
生物-生化与分子生物学
CiteScore
6.40
自引率
0.00%
发文量
139
审稿时长
30 days
期刊介绍:
BBA General Subjects accepts for submission either original, hypothesis-driven studies or reviews covering subjects in biochemistry and biophysics that are considered to have general interest for a wide audience. Manuscripts with interdisciplinary approaches are especially encouraged.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


