Harnessing marine natural products to inhibit PAD4 triple mutant: A structure-based virtual screening approach for rheumatoid arthritis therapy
IF 3.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Peptidylarginine deiminase type4 (PAD4) is a pivotal pro-inflammatory protein within the human immune system, intricately involved in both inflammatory processes and immune responses. Its role extends to the generation of diverse immune cell types, including T cells, B cells, natural killer cells, and dendritic cells. PAD4 has recently garnered attention due to its association with a spectrum of inflammatory and autoimmune disorders, notably rheumatoid arthritis (RA). Mutations in the PAD4 gene, leading to the conversion of arginine to citrulline, have emerged as significant factors in the pathogenesis of RA and related conditions. As a calcium-dependent enzyme, PAD4 is central to the citrullination process, a crucial post-translational modification implicated in disease pathophysiology. Its critical role in autoimmune disorders and inflammation makes PAD4 a prime candidate for therapeutic intervention in RA. Inhibiting PAD4 presents a promising avenue for mitigating inflammatory responses and curtailing joint degradation and impairment. To explore its therapeutic potential, a structure-based virtual screening (SBVS) approach was employed, harnessing an array of marine natural products (MNPs) sourced from databases such as CMNPD, MNPD, and Seaweed. Notably, MNPD10752, CMNPD12680, and CMNPD2751 emerged as potential hit molecules, exhibiting adherence to essential pharmacokinetic properties and favorable toxicity profiles. Quantum mechanics studies using density functional theory (DFT) calculations revealed the inhibitory potential of these identified natural products. Further structural elucidation through molecular dynamics simulations (MDS) and principal component-based free energy landscape (FEL) analysis shed light on the stability of MNP-bound PAD4 complexes. In conclusion, this computational study serves as a stepping stone for further experimental evaluation, aiming to explore the potential of MNPs in addressing PAD4-related human pathologies.
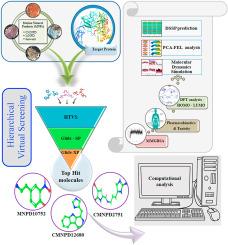
利用海洋天然产物抑制 PAD4 三重突变体:基于结构的类风湿关节炎治疗虚拟筛选方法。
肽基精氨酸脱氨酶 4 型(PAD4)是人体免疫系统中一种关键的促炎症蛋白,密切参与炎症过程和免疫反应。它的作用延伸到多种免疫细胞类型的生成,包括 T 细胞、B 细胞、自然杀伤细胞和树突状细胞。最近,PAD4 因其与一系列炎症和自身免疫性疾病,尤其是类风湿性关节炎(RA)的关联而备受关注。PAD4 基因突变会导致精氨酸转化为瓜氨酸,已成为类风湿性关节炎及相关疾病发病机制的重要因素。作为一种钙依赖性酶,PAD4 是瓜氨酸化过程的核心,而瓜氨酸化是一种关键的翻译后修饰,与疾病的病理生理学有关。它在自身免疫性疾病和炎症中的关键作用使 PAD4 成为对 RA 进行治疗干预的主要候选对象。抑制 PAD4 为减轻炎症反应、遏制关节退化和损伤提供了一条很有前景的途径。为了探索其治疗潜力,我们采用了一种基于结构的虚拟筛选(SBVS)方法,利用了来自 CMNPD、MNPD 和 Seaweed 等数据库的一系列海洋天然产物(MNPs)。值得注意的是,MNPD10752、CMNPD12680 和 CMNPD2751 成为潜在的命中分子,它们表现出基本的药代动力学特性和良好的毒性特征。利用密度泛函理论(DFT)计算进行的量子力学研究揭示了这些已确定的天然产物的抑制潜力。通过分子动力学模拟 (MDS) 和基于主成分的自由能谱 (FEL) 分析进一步阐明了 MNP 结合 PAD4 复合物的稳定性。总之,这项计算研究为进一步的实验评估奠定了基础,旨在探索 MNPs 解决 PAD4 相关人类病症的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


