Mechanistic analysis of thermal stability in a novel thermophilic polygalacturonase MlPG28B derived from the marine fungus Mucor lusitanicus
IF 7.7
1区 化学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
International Journal of Biological Macromolecules
Pub Date : 2024-09-24
DOI:10.1016/j.ijbiomac.2024.136007
引用次数: 0
Abstract
In this study, heterologous MlPG28B expression was obtained by cloning the Mucor lusitanicus gene screened from a marine environment. The enzyme activity of MlPG28B was maximum at 60 °C, 30 % of the enzyme activity was retained after incubation at 100 °C for 30 min, and enzyme activity was still present after 60 min incubation, one of the best thermostable polygalacturonases characterized until now. The high-purity oligosaccharide standards (DP2–DP7) were prepared with polygalacturonic acid as a substrate. Kinetic parameters showed that MlPG28B at the optimum temperature has a low Km value (3055 ± 1104 mg/L), indicating high substrate affinity. Sequence alignment analysis inferred key residues Cys276, Cys284, Lys107, and Gln237 for MlPG28B thermal stability. Molecular docking and molecular dynamics simulation results indicated that MlPG28B has flexible T1 and T3 loops conducive to substrate recognition, binding, and catalysis and forms a hydrogen bond to the substrate by a highly conserved residue Asn161 in the active-site cleft. Based on site-directed mutation results, the five residues are key in determining MlPG28B thermal stability. Therefore, MlPG28B is a promising candidate for industrial enzymes in feed preparation.
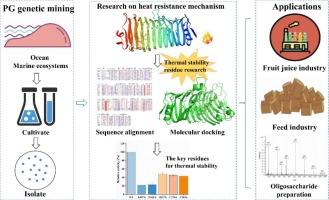
从海洋真菌 Mucor lusitanicus 中提取的新型嗜热型聚半乳糖醛酸酶 MlPG28B 的热稳定性机理分析。
本研究通过克隆从海洋环境中筛选的 Mucor lusitanicus 基因,获得了异源 MlPG28B 表达。MlPG28B 的酶活性在 60 ℃ 时达到最大,100 ℃ 培养 30 分钟后仍能保持 30% 的酶活性,培养 60 分钟后仍有酶活性,是迄今为止热稳定性最好的聚半乳糖醛酸酶之一。以聚半乳糖醛酸为底物制备了高纯度寡糖标准品(DP2-DP7)。动力学参数表明,MlPG28B 在最适温度下具有较低的 Km 值(3055 ± 1104 mg/L),表明其具有较高的底物亲和力。序列比对分析推断出 Cys276、Cys284、Lys107 和 Gln237 是 MlPG28B 热稳定性的关键残基。分子对接和分子动力学模拟结果表明,MlPG28B具有灵活的T1和T3环,有利于底物的识别、结合和催化,并通过活性位点裂隙中高度保守的残基Asn161与底物形成氢键。根据定点突变结果,这五个残基是决定 MlPG28B 热稳定性的关键。因此,MlPG28B 是一种很有前途的饲料制备工业酶。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
13.70
自引率
9.80%
发文量
2728
审稿时长
64 days
期刊介绍:
The International Journal of Biological Macromolecules is a well-established international journal dedicated to research on the chemical and biological aspects of natural macromolecules. Focusing on proteins, macromolecular carbohydrates, glycoproteins, proteoglycans, lignins, biological poly-acids, and nucleic acids, the journal presents the latest findings in molecular structure, properties, biological activities, interactions, modifications, and functional properties. Papers must offer new and novel insights, encompassing related model systems, structural conformational studies, theoretical developments, and analytical techniques. Each paper is required to primarily focus on at least one named biological macromolecule, reflected in the title, abstract, and text.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


