Long-term follow-up of the TRED-HF trial: Implications for therapy in patients with dilated cardiomyopathy and heart failure remission
Abstract
Aims
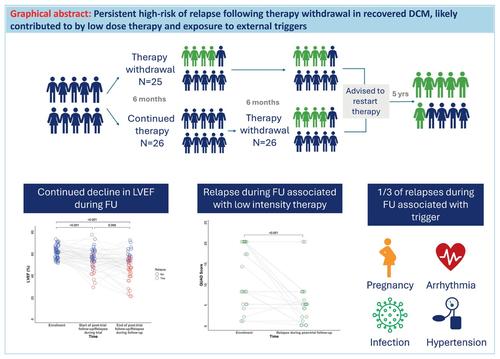
In TRED-HF, 40% of patients with recovered dilated cardiomyopathy (DCM) relapsed in the short term after therapy withdrawal. This follow-up investigates the longer-term effects of therapy withdrawal.
Methods and results
TRED-HF was a randomized trial investigating heart failure therapy withdrawal in patients with recovered DCM over 6 months. Those randomized to continue therapy subsequently withdrew treatment between 6 and 12 months. Participants were recommended to restart therapy post-trial and were followed until May 2023. Clinical outcomes are reported in a non-randomized fashion from enrolment and from the end of the trial. The primary outcome was relapse defined as ≥10% reduction in left ventricular ejection fraction to <50%, doubling in N-terminal pro-B-type natriuretic peptide to >400 ng/L, or clinical features of heart failure. From enrolment to the last follow-up (median 6 years, interquartile range 6–7), 33 of 51 patients (65%) relapsed. The 5-year relapse rate from enrolment was 61% (95% confidence interval [CI] 45–73) and from the end of the trial was 39% (95% CI 19–54). Of 20 patients who relapsed during the trial, nine had a recurrent relapse during follow-up. Thirteen relapsed for the first time after the trial; seven had restarted low intensity therapy, four had not restarted therapy and two did not have therapy withdrawn. The mean intensity of therapy was lower after the trial compared to enrolment (mean difference −6 [−8 to −4]; p < 0.001). One third of relapses during follow-up had identifiable triggers (arrhythmia [n = 4], pregnancy [n = 1], hypertension [n = 1], infection [n = 1]). Corrected atrial fibrillation was associated with reduced risk of relapse (hazard ratio 0.33, 95% CI 0.12–0.96; p = 0.042).
Conclusions
The risk of relapse in the 5 years following the TRED-HF trial remained high. Restarting lower doses of heart failure medications at the end of the trial, external triggers and disease progression are likely to have contributed to relapse.


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


