MEF2C Alleviates Postoperative Cognitive Dysfunction by Repressing Ferroptosis
Abstract
Background
Ferroptosis, a form of programmed cell death featured by lipid peroxidation, has been proposed as a potential etiology for postoperative cognitive dysfunction (POCD). Myocyte-specific enhancer factor 2C (MEF2C), a transcription factor expressed in various brain cell types, has been implicated in cognitive disorders. This study sought to ascertain whether MEF2C governs postoperative cognitive capacity by affecting ferroptosis.
Methods
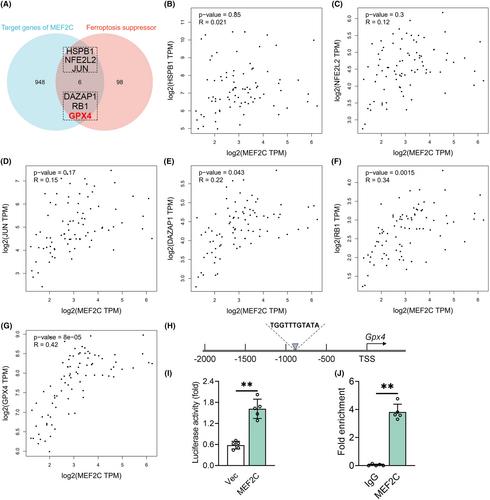
Transcriptomic analysis of public data was used to identify MEF2C as a candidate differentially expressed gene in the hippocampus of POCD mice. The POCD mouse model was established via aseptic laparotomy under isoflurane anesthesia after treatment with recombinant adeno-associated virus 9 (AAV9)-mediated overexpression of MEF2C and/or the glutathione peroxidase 4 (GPX4) inhibitor RSL3. Cognitive performance, Nissl staining, and ferroptosis-related parameters were assessed. Dual-luciferase reporter gene assays and chromatin immunoprecipitation assays were implemented to elucidate the mechanism by which MEF2C transcriptionally activates GPX4.
Results
MEF2C mRNA and protein levels decreased in the mouse hippocampus following anesthesia and surgery. MEF2C overexpression ameliorated postoperative memory decline, hindered lipid peroxidation and iron accumulation, and enhanced antioxidant capacity, which were reversed by RSL3. Additionally, MEF2C was found to directly bind to the Gpx4 promoter and activate its transcription.
Conclusions
Our findings suggest that MEF2C may be a promising therapeutic target for POCD through its negative modulation of ferroptosis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


