Early growth response 1 exacerbates thoracic aortic aneurysm and dissection of mice by inducing the phenotypic switching of vascular smooth muscle cell through the activation of Krüppel-like factor 5
Abstract
Aim
Vascular smooth muscle cell (VSMC) phenotypic switching has been reported to regulate vascular function and thoracic aortic aneurysm and dissection (TAAD) progression. Early growth response 1 (Egr1) is associated with the differentiation of VSMCs. However, the mechanisms through which Egr1 participates in the regulation of VSMCs and progression of TAAD remain unknown. This study aimed to investigate the role of Egr1 in the phenotypic switching of VSMCs and the development of TAAD.
Methods
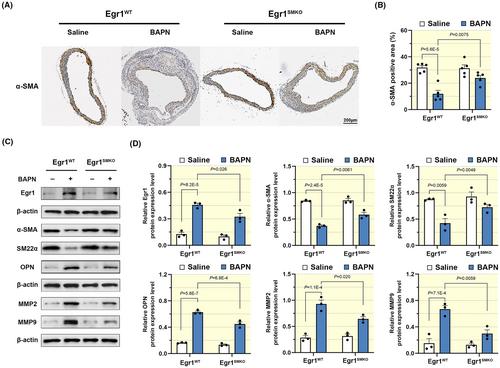
Wild-type C57BL/6 and SMC-specific Egr1-knockout mice were used as experimental subjects and fed β-aminopropionitrile for 4 weeks to construct the TAAD model. Ultrasound and aortic staining were performed to examine the pathological features in thoracic aortic tissues. Transwell, wound healing, CCK8, and immunofluorescence assays detected the migration and proliferation of synthetic VSMCs. Egr1 was directly bound to the promoter of Krüppel-like factor 5 (KLF5) and promoted the expression of KLF5, which was validated by JASPAR database and dual-luciferase reporter assay.
Results
Egr1 expression increased and was partially co-located with VSMCs in aortic tissues of mice with TAAD. SMC-specific Egr1 deficiency alleviated TAAD and inhibited the phenotypic switching of VSMC. Egr1 knockdown prevented the phenotypic switching of VSMCs and subsequently suppressed the migration and proliferation of synthetic VSMCs. The inhibitory effects of Egr1 deficiency on VSMCs were blunted once KLF5 was overexpressed.
Conclusion
Egr1 aggravated the development of TAAD by promoting the phenotypic switching of VSMCs via enhancing the transcriptional activation of KLF5. These results suggest that inhibition of SMC-specific Egr1 expression is a promising therapy for TAAD.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


