Stereoselective synthetic approach toward β-trifluoromethyl vinyl ethers and diethers via reaction of (E)-1,2-dichloro-3,3,3-trifluoroprop-1-ene with phenols†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A convenient method for synthesizing β-trifluoromethyl vinyl ethers and diethers through the base-mediated C–O coupling of (E)-1,2-dichloro-3,3,3-trifluoroprop-1-ene and phenols has been developed. Remarkably, the present process shows perfect regioselective and stereoselective yield of the Z/E isomers for β-trifluoromethyl vinyl ethers with high efficiency. Additionally, β-trifluoromethyl vinyl diethers with identical/diverse phenoxy groups were also obtained and the regulation of the product configuration was achieved. These reactions feature transition-metal-free conditions, wide substrate scope, and atom economy.
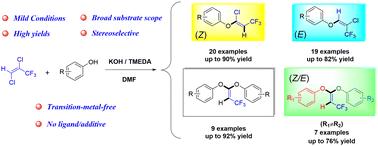
通过(E)-1,2-二氯-3,3,3-三氟丙烯与苯酚的反应,立体选择性合成 β-三氟甲基乙烯基醚和二醚。
通过碱介导的 (E)-1,2-dichloro-3,3,3-trifluoroprop-1-ene 和苯酚的 C-O 偶联,开发出了一种合成 β-三氟甲基乙烯基醚和二醚的简便方法。值得注意的是,本工艺对 β-三氟甲基乙烯基醚的 Z/E 异构体具有完美的区域选择性和立体选择性,而且效率很高。此外,还获得了具有相同/不同苯氧基的 β-三氟甲基乙烯基二醚,并实现了对产物构型的调节。这些反应具有无过渡金属条件、底物范围广和原子经济等特点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


