DeSUMOylation of RBMX regulates exosomal sorting of cargo to promote renal tubulointerstitial fibrosis in diabetic kidney disease
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Diabetic kidney disease (DKD) has become the primary cause of chronic renal failure in China, and renal tubulointerstitial fibrosis plays a central role in DKD progression. Urinary exosomes, which reflect kidney changes, are largely influenced by RNA-binding proteins (RBPs) in their miRNA content.
Objectives
Our research aimed to determine the effect of the RNA-binding protein RBMX on exosomal miRNA in DKD.
Methods
We introduced a higher level of Rbmx into diabetic mice using an adenoassociated virus and isolated exosomes from their kidney tissue through advanced centrifugation techniques and specialized kits. We then conducted a series of tests, including qRT-PCR, Western blot, MitoSOX, ATP luminescence, coimmunoprecipitation, SUMOylation assays, RNA immunoprecipitation, and confocal microscopy.
Results
RBMX is found in higher levels in DKD and contributes to worsening kidney fibrosis, mitochondrial damage, and miRNA mismanagement in exosomes. It specifically binds with miR-26a, miR-23c, and miR-874 within the exosomes. This dysfunction may be linked to changes in RBMX SUMOylation. These miRNAs seem to protect against mitochondrial damage in kidney cells by targeting CERS6.
Conclusion
DeSUMOylation of RBMX plays a crucial role in determining the makeup of miRNAs in kidney cell exosomes, impacting the protective miRNAs which regulate mitochondrial damage through their interaction with CERS6 mRNA, ultimately affecting mitochondrial health in DKD.
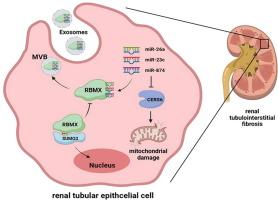
RBMX的去SUMOylation调节货物的外泌体分拣,从而促进糖尿病肾病的肾小管间质纤维化。
简介糖尿病肾病(DKD)已成为中国慢性肾功能衰竭的主要病因,而肾小管间质纤维化在DKD进展中起着核心作用。反映肾脏变化的尿液外泌体的miRNA含量主要受RNA结合蛋白(RBPs)的影响:我们的研究旨在确定 RNA 结合蛋白 RBMX 对外泌体 miRNA 在 DKD 中的影响:方法:我们利用腺相关病毒将较高水平的Rbmx引入糖尿病小鼠体内,并通过先进的离心技术和专用试剂盒从其肾组织中分离出外泌体。然后,我们进行了一系列检测,包括 RT-qPCR、Western 印迹、MitoSOX、ATP 发光、共免疫沉淀、SUMOylation 检测、RNA 免疫沉淀和共聚焦显微镜:结果:RBMX在DKD中含量较高,导致肾脏纤维化恶化、线粒体损伤和外泌体中miRNA管理不善。它能与外泌体中的 miR-26a、miR-23c 和 miR-874 特异性结合。这种功能障碍可能与 RBMX SUMOylation 的变化有关。这些 miRNA 似乎通过靶向 CERS6 保护肾细胞免受线粒体损伤:RBMX的去SUMOylation在决定肾细胞外泌体中miRNA的组成方面起着至关重要的作用,影响着保护性miRNA,而保护性miRNA通过与CERS6 mRNA相互作用来调节线粒体损伤,最终影响DKD患者的线粒体健康。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


