A novel effect of sulforaphane on promoting mouse granulosa cells proliferation via the NRF2–TKT pathway
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Granulosa cells (GCs) is essential for maintaining follicular development. Follicle-stimulating Hormone (FSH) has been demonstrated to effectively promote GCs proliferation, driving the establishment of various superovulation techniques for animal husbandry. However, these techniques face challenges, such as high costs, hormonal imbalances, and an increased risk of early ovarian dysfunction. Therefore, it is important to investigate new methods to improve GCs proliferation.
Objectives
This study aimed to investigate the effect of sulforaphane (SFN) on ovarian GCs proliferation and the underlying mechanisms.
Methods
A comparative transcriptomic analysis of ovaries from the control, SFN, and FSH groups was conducted to identify the primary factors contributing to high proliferative capacity. The role of SFN in the regulation of cell proliferation has been examined in mouse ovarian GCs. Gene interference, overexpression, CUT&TAG technology, and transcriptome analyses were performed to elucidate the underlying mechanisms of the nuclear factor E2-related factor 2 (NRF2)–transketolase (TKT) axis in mediating GCs proliferation.
Results
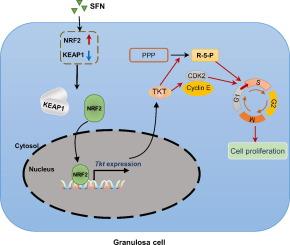
Our research revealed a previously unknown function of SFN, an isothiocyanate of plant origin that is prevalent in cruciferous vegetables, in facilitating the proliferation of mouse ovarian GCs. The efficacy of SFN in enhancing GCs proliferation is similar to that of FSH. At the mechanistic level, SFN promotes NRF2 to transport to the nucleus, which subsequently activates the key enzyme of the non-oxidative pentose phosphate pathway TKT. This activation is instrumental in generating ribose 5-phosphate, a critical precursor for amino acid and nucleotide biosynthesis that underpins the proliferation of GCs.
Conclusion
Collectively, our findings delineate a novel pathway by which SFN, through the NRF2-TKT axis, enhances the nucleotide pool and thereby supports the proliferation of mouse GCs, presenting novel avenues for exploration in reproductive biology and agricultural sciences.
莱菔硫烷通过 NRF2-TKT 通路促进小鼠颗粒细胞增殖的新作用
简介颗粒细胞(GCs)对维持卵泡发育至关重要。卵泡刺激素(FSH)已被证明能有效促进颗粒细胞增殖,从而推动了各种动物超排卵技术的建立。然而,这些技术面临着高成本、激素失衡和早期卵巢功能障碍风险增加等挑战。因此,研究改善GCs增殖的新方法非常重要:本研究旨在探讨莱菔硫烷(SFN)对卵巢GCs增殖的影响及其内在机制:方法:对对照组、SFN组和FSH组的卵巢进行转录组学比较分析,以确定导致高增殖能力的主要因素。在小鼠卵巢GCs中研究了SFN在细胞增殖调控中的作用。通过基因干扰、过表达、CUT&TAG技术和转录组分析,阐明了核因子E2相关因子2(NRF2)-转酮酶(TKT)轴在介导GCs增殖中的潜在机制:我们的研究揭示了SFN在促进小鼠卵巢GCs增殖方面之前未知的功能,SFN是一种源自植物的异硫氰酸盐,普遍存在于十字花科蔬菜中。SFN在促进GCs增殖方面的功效与FSH相似。在机理层面,SFN促进NRF2向细胞核运输,随后激活非氧化磷酸戊糖途径的关键酶TKT。这种激活有助于产生 5-磷酸核糖,这是氨基酸和核苷酸生物合成的关键前体,是 GCs 增殖的基础:总之,我们的研究结果描述了一种新的途径,SFN 通过 NRF2-TKT 轴增强核苷酸池,从而支持小鼠 GCs 的增殖,为生殖生物学和农业科学提供了新的探索途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


