Biomimetic copper-containing nanogels for imaging-guided tumor chemo-chemodynamic-immunotherapy
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
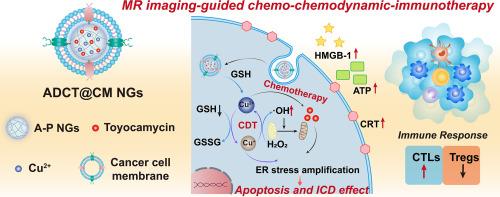
Developing multifunctional nanoplatforms to comprehensively modulate the tumor microenvironment and enhance diagnostic and therapeutic outcomes still remains a great challenge. Here, we report the facile construction of a multivariate nanoplatform based on cancer cell membrane (CM)-encapsulated redox-responsive poly(N-vinylcaprolactam) (PVCL) nanogels (NGs) co-loaded with Cu(II) and chemotherapeutic drug toyocamycin (Toy) for magnetic resonance (MR) imaging-guided combination tumor chemodynamic therapy/chemoimmunotherapy. We show that redox-responsive PVCL NGs formed through precipitation polymerization can be aminated, conjugated with 3,4-dihydroxyhydrocinnamic acid for Cu(II) complexation, physically loaded with Toy, and finally camouflaged with CMs. The created ADCT@CM NGs with an average size of 113.0 nm are stable under physiological conditions and can efficiently release Cu(II) and Toy under tumor microenvironment with a high level of glutathione. Meanwhile, the developed NGs are able to enhance cancer cell oxidative stress and endoplasmic reticulum stress by synergizing the effects of chemodynamic therapy mediated by Cu-based Fenton-like reaction and Toy-mediated chemotherapy, thereby triggering significant immunogenic cell death (ICD). In a melanoma mouse model, the NGs show potent immune activation effects to reinforce tumor therapeutic efficacy through ICD induction and immune modulation including high levels of immune cytokine secretion, increased tumor infiltration of CD8+ cytotoxic T cells, and reduced tumor infiltration of regulatory T cells. With the CM coating and Cu(II) loading, the developed NG platform demonstrates homologous tumor targeting and T1-weighted MR imaging, hence providing a general biomimetic NG platform for ICD-facilitated tumor theranostic nanoplatform.
Statement of significance
Developing multifunctional nanoplatforms to comprehensively modulate the tumor microenvironment (TME) and enhance theranostic outcomes remains a challenge. Here, a cancer cell membrane (CM)-camouflaged nanoplatform based on aminated poly(N-vinylcaprolactam) nanogels (NGs) co-loaded with Cu(II) and toyocamycin (Toy) was prepared for magnetic resonance (MR) imaging-guided combination tumor chemodynamic therapy/chemoimmunotherapy. The tumor targeting specificity and efficient TME-triggered release of Cu(II) and Toy could enhance tumor cell oxidative stress and endoplasmic reticulum stress by synergizing the effects of chemodynamic therapy mediated by Cu-based Fenton-like reaction and Toy-mediated chemotherapy, respectively, thereby leading to significant immunogenic cell death (ICD) and immune response. With the CM coating and Cu(II) loading, the developed NG platform also demonstrates good T1-weighted tumor MR imaging performance. Hence, this study provides a general biomimetic NG platform for ICD-facilitated tumor theranostics.
用于成像引导的肿瘤化学-化学动力-免疫疗法的仿生含铜纳米凝胶
开发多功能纳米平台以全面调节肿瘤微环境并提高诊断和治疗效果仍然是一项巨大挑战。在这里,我们报告了一种基于癌细胞膜(CM)封装的氧化还原响应聚(N-乙烯基己内酰胺)(PVCL)纳米凝胶(NGs)的多元纳米平台的简易构建,该平台共负载了铜(II)和化疗药物玩具霉素(Toy),用于磁共振(MR)成像引导的肿瘤化学动力疗法/血液免疫疗法联合治疗。我们的研究表明,通过沉淀聚合形成的具有氧化还原反应的 PVCL NG 可被胺化,与 3,4-二羟基氢肉桂酸共轭以络合 Cu(II),物理负载 Toy,最后与 CM 伪装在一起。所制备的 ADCT@CM NG 平均尺寸为 113.0 nm,在生理条件下非常稳定,并能在谷胱甘肽含量较高的肿瘤微环境中有效释放 Cu(II) 和 Toy。同时,所开发的 NGs 能够通过协同铜基 Fenton-like 反应介导的化学动力疗法和 Toy 介导的化疗的效果,增强癌细胞氧化应激和内质网应激,从而引发显著的免疫原性细胞死亡(ICD)。在黑色素瘤小鼠模型中,NGs 通过诱导 ICD 和免疫调节(包括高水平的免疫细胞因子分泌、CD8+ 细胞毒性 T 细胞的肿瘤浸润增加以及调节性 T 细胞的肿瘤浸润减少)显示出强大的免疫激活效应,从而加强了肿瘤疗效。在 CM 涂层和 Cu(II) 负载的作用下,所开发的 NG 平台实现了同源肿瘤靶向和 T1 加权 MR 成像,从而为 ICD 促成的肿瘤治疗纳米平台提供了一种通用的生物仿生 NG 平台。意义说明:开发多功能纳米平台以全面调节肿瘤微环境(TME)并提高治疗效果仍然是一项挑战。本文制备了一种基于氨基化聚(N-乙烯基己内酰胺)纳米凝胶(NGs)的癌症细胞膜(CM)伪装纳米平台,该纳米凝胶共同负载了铜(II)和玩具霉素(Toy),可用于磁共振(MR)成像引导的肿瘤化学动力疗法/血液免疫疗法联合疗法。Cu(II)和Toy的肿瘤靶向特异性和高效的TME触发释放可增强肿瘤细胞氧化应激和内质网应激,分别协同Cu基Fenton样反应介导的化学动力疗法和Toy介导的化疗的效果,从而导致显著的免疫原性细胞死亡(ICD)和免疫反应。在 CM 涂层和 Cu(II) 负载的作用下,所开发的 NG 平台还具有良好的 T1 加权肿瘤磁共振成像性能。因此,这项研究为 ICD 促成的肿瘤治疗提供了一种通用的生物仿生 NG 平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


