An injectable thermosensitive hydrogel delivering M2 macrophage-derived exosomes alleviates osteoarthritis by promoting synovial lymphangiogenesis
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Osteoarthritis (OA) is a prevalent chronic degenerative disease affecting millions worldwide, with current treatment measures lacking efficacy in slowing disease progression. The synovial lymphatic system (SLS) has emerged as a crucial player in OA pathogenesis, with compromised drainage function contributing to disease advancement. Lymphatic endothelial cells (LECs) within the SLS are influenced by synovial macrophages, whose precise impact on LEC function remains unclear. Exosomes released by macrophages may serve as mediators of this interaction, with potential implications for OA progression. Here, we propose that polarized macrophages modulate LEC activity via exosome release in synovial tissue, with M2 macrophage-derived exosomes (M2Exo) promoting LEC proliferation, migration, and lymphangiogenesis, potentially offering a therapeutic avenue for OA. Moreover, we developed an injectable thermosensitive hydrogel with the characteristic of sustained release of M2Exo for alleviating OA. The hydrogel was prepared by dynamically linking hyaluronic acid (HA) and Pluronic F-127 and loading M2Exo, termed as M2Exo loaded HP hydrogel. The in vitro and in vivo experiments showed that M2Exo loaded HP hydrogel exhibits a controlled release profile of exosomes, thereby efficaciously fostering synovial lymphangiogenesis and enhancing synovial lymphatic drainage functionality under OA conditions, thus alleviating OA progression, and providing promising insights into OA therapeutic strategies.
Statement of significance
Osteoarthritis (OA) is a widespread degenerative disease with limited effective treatments to halt its progression. This research highlights the critical role of the synovial lymphatic system (SLS) in OA, focusing on how macrophage-derived exosomes influence lymphatic endothelial cell (LEC) function. We propose that M2 macrophage-derived exosomes (M2Exo) enhance LEC activity, promoting lymphangiogenesis, and offering a therapeutic approach for OA. Furthermore, we developed an injectable thermosensitive hydrogel (M2Exo loaded HP hydrogel) for sustained M2Exo release. Our in vitro and in vivo experiments demonstrate that this hydrogel supports synovial lymphangiogenesis and improves lymphatic drainage, effectively alleviating OA progression. This study presents significant advancements in OA therapy, offering new insights into its management.
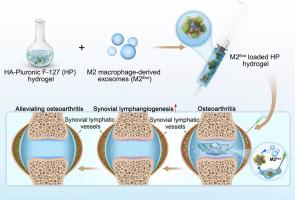
一种可注射的热敏性水凝胶可通过促进滑膜淋巴管生成输送源自 M2 巨噬细胞的外泌体,从而缓解骨关节炎。
骨关节炎(OA)是一种普遍存在的慢性退行性疾病,影响着全球数百万人,目前的治疗措施在减缓疾病进展方面缺乏疗效。滑膜淋巴系统(SLS)已成为骨关节炎发病机制中的关键因素,其引流功能受损会导致疾病恶化。滑膜淋巴系统中的淋巴内皮细胞(LECs)受到滑膜巨噬细胞的影响,而巨噬细胞对LEC功能的确切影响尚不清楚。巨噬细胞释放的外泌体可能是这种相互作用的介质,对 OA 的进展具有潜在影响。在这里,我们提出极化的巨噬细胞通过在滑膜组织中释放外泌体来调节LEC的活性,M2巨噬细胞衍生的外泌体(M2Exo)可促进LEC的增殖、迁移和淋巴管生成,从而为OA提供潜在的治疗途径。此外,我们还开发了一种具有持续释放 M2Exo 特性的可注射热敏水凝胶,用于缓解 OA。这种水凝胶是通过动态连接透明质酸(HA)和Pluronic F-127并负载M2Exo制备而成的,称为负载M2Exo的HP水凝胶。体外和体内实验表明,M2Exo负载的HP水凝胶具有外泌体可控释放特性,因此能有效促进OA条件下的滑膜淋巴管生成,增强滑膜淋巴引流功能,从而缓解OA进展,并为OA治疗策略提供了有前景的见解。意义说明:骨关节炎(OA)是一种广泛存在的退行性疾病,但阻止其恶化的有效治疗方法却很有限。这项研究强调了滑膜淋巴系统(SLS)在骨关节炎中的关键作用,重点研究了巨噬细胞衍生的外泌体如何影响淋巴内皮细胞(LEC)的功能。我们提出,M2巨噬细胞衍生的外泌体(M2Exo)可增强淋巴内皮细胞的活性,促进淋巴管生成,为OA提供一种治疗方法。此外,我们还开发了一种可注射的热敏水凝胶(M2Exo负载HP水凝胶),用于持续释放M2Exo。我们的体外和体内实验证明,这种水凝胶可支持滑膜淋巴管生成并改善淋巴引流,从而有效缓解 OA 进展。这项研究在治疗 OA 方面取得了重大进展,为 OA 的治疗提供了新的思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


