Graft survival of major histocompatibility complex deficient stem cell-derived retinal cells
IF 5.4
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
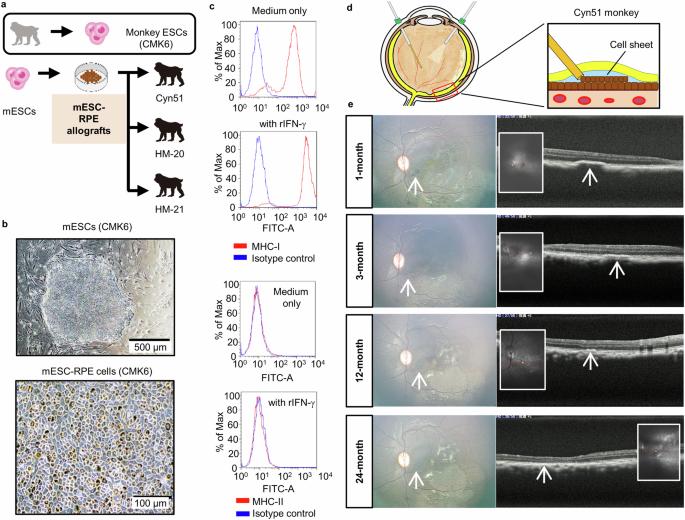
Gene editing of immunomodulating molecules is a potential transplantation strategy to control immune rejection. As we noticed the successful transplantation of retinal pigment epithelium (RPE) derived from embryonic stem cells of a cynomolgus monkey that accidentally lacked MHC class II (MHC-II) molecules, we hypothesized immune rejection could be evaded by suppressing MHC-II. Gene editing by the Crispr/Cas9 system was performed in induced pluripotent stem cells derived from a cynomolgus monkey (miPSCs) for targeted deletion of the gene coding class II MHC trans-activator (CIITA). Then the CIITA-knocked out miPSCs were differentiated into RPE cells to generate miPSC-derived MHC-II knockout RPE. The MHC-II knockout or wild-type RPEs were transplanted into the eyes of healthy cynomolgus monkeys. All monkeys used in this study were male. Here we show when MHC-II knockout RPE are transplanted into monkey eyes, they show suppressed immunogenicity with no infiltration of inflammatory cells, leading to successful engraftment. Our results reasonably evidence the efficacy of MHC-II knockout iPSC-RPE transplants for clinical application. Transplantation of healthy cells can be used to treat irreversibly damaged organs. However, a concern is that the transplanted cells will be rejected by the immune system. Generally, the immune system protects our body when unknown materials invade. But this is undesirable during cell transplantation as the transplanted cells are often eliminated by the host’s immune cells. We demonstrated in monkeys that deletion of part of the immune system in cells prior to transplantation reduced the amount of immune system activity following transplantation. Using similar strategies in the future could enable cell transplants to be used more successfully in humans, making cell transplantation therapy safer and applicable to a wider number of patients. Ishida et al. transplant Crispr/Cas9 gene edited MHC-II knockout or wild-type retinal pigment epithelium into cynomolgus monkey eyes. MHC-II knockout RPE engraft successfully with no infiltration of inflammatory cells.
主要组织相容性复合体缺陷干细胞衍生视网膜细胞的移植存活。
背景:免疫调节分子的基因编辑是一种控制免疫排斥反应的潜在移植策略。我们注意到,从一只意外缺乏MHC-II类(MHC-II)分子的野猴胚胎干细胞中提取的视网膜色素上皮(RPE)移植获得了成功,因此我们假设可以通过抑制MHC-II来避免免疫排斥反应:方法:通过Crispr/Cas9系统对诱导多能干细胞(miPSCs)进行基因编辑,定向删除编码MHC II类反式激活剂(CIITA)的基因。然后将CIITA基因敲除的miPSCs分化成RPE细胞,生成miPSC衍生的MHC-II基因敲除RPE。将 MHC-II 基因剔除或野生型 RPE 移植到健康猴的眼睛中。本研究中使用的所有猴子均为雄性:结果:我们在此表明,当将 MHC-II 基因敲除的 RPE 移植到猴眼中时,它们显示出抑制的免疫原性,没有炎症细胞浸润,从而导致成功的移植:我们的研究结果合理地证明了 MHC-II 基因敲除 iPSC-RPE 移植在临床应用中的有效性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


