Remote haemodynamic-guided heart failure management in France: Results from the CardioMEMS HF System Post-Market Study (COAST) French cohort
IF 2.3
3区 医学
Q2 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Background
Previous studies have demonstrated the benefit of a haemodynamic-guided management strategy with the CardioMEMS™ HF System. No data from French patients have been published.
Aims
To analyse the feasibility, safety and clinical benefit of the CardioMEMS™ HF System in 103 French patients included in the CardioMEMS HF System Post-Market Study (COAST).
Methods
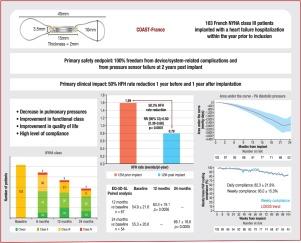
Prospective open-label cohort of New York Heart Association class III patients with at least one heart failure hospitalization in the 12 months before enrolment, regardless of left ventricular ejection fraction. The primary safety endpoints assessed the freedom from device/system-related complications and from pressure sensor failure at 2 years after implantation. The primary efficacy endpoint was evaluated comparing the rate of heart failure hospitalization during the year before and the year after implantation.
Results
At 2 years, there were no device/system-related complications or pressure sensor failures (P < 0.0001). There were 179 heart failure hospitalizations in the year before implantation compared with 79 in the year after implantation (risk reduction 50.3%; rate ratio 0.50, 95% confidence interval 0.38–0.66; P < 0.0001). During the 2 years of follow-up, pulmonary artery pressures were lowered significantly (mean pulmonary artery pressure –3.7 ± 6.3 mmHg; P < 0.0001), with a significant improvement in functional class and quality of life.
Conclusions
In the French cohort of the COAST study, we have demonstrated that the CardioMEMS™ HF System is a reliable device, with no device/system-related complications or pressure sensor failures. Patients in this open-label cohort had a significant reduction in pulmonary artery pressures, with an improvement in New York Heart Association classification and quality of life, and a 50% reduction in the heart failure hospitalization rate in the year following implantation compared with the previous year.
法国的远程血流动力学指导心衰管理:CardioMEMS HF 系统上市后研究 (COAST) 法国队列的结果。
背景:先前的研究表明,使用 CardioMEMS™ HF 系统进行血流动力学指导管理策略具有益处。目的:分析 CardioMEMS™ HF 系统在 103 名法国患者中的可行性、安全性和临床获益,这些患者被纳入 CardioMEMS HF 系统上市后研究 (COAST):前瞻性开放标签队列:纽约心脏协会 III 级患者,入组前 12 个月内至少有一次心力衰竭住院经历,不考虑左心室射血分数。主要安全性终点是评估植入设备/系统相关并发症的发生率以及植入后两年压力传感器故障的发生率。主要疗效终点是比较植入前一年和植入后一年的心衰住院率:结果:植入 2 年后,没有出现装置/系统相关并发症或压力传感器故障(PC 结论:在法国的 AOC 研究队列中,没有出现装置/系统相关并发症或压力传感器故障:在 COAST 研究的法国队列中,我们证明了 CardioMEMS™ 高频系统是一种可靠的设备,没有出现设备/系统相关并发症或压力传感器故障。该开放标签队列中的患者肺动脉压力显著降低,纽约心脏协会分级和生活质量得到改善,植入后一年的心衰住院率与前一年相比降低了 50%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of Cardiovascular Diseases
医学-心血管系统
CiteScore
4.40
自引率
6.70%
发文量
87
审稿时长
34 days
期刊介绍:
The Journal publishes original peer-reviewed clinical and research articles, epidemiological studies, new methodological clinical approaches, review articles and editorials. Topics covered include coronary artery and valve diseases, interventional and pediatric cardiology, cardiovascular surgery, cardiomyopathy and heart failure, arrhythmias and stimulation, cardiovascular imaging, vascular medicine and hypertension, epidemiology and risk factors, and large multicenter studies. Archives of Cardiovascular Diseases also publishes abstracts of papers presented at the annual sessions of the Journées Européennes de la Société Française de Cardiologie and the guidelines edited by the French Society of Cardiology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


