Catheter-based renal denervation in the treatment of arterial hypertension: An expert consensus statement on behalf of the French Society of Hypertension (SFHTA), French Society of Radiology (SFR), French Society of Interventional Cardiology (GACI), French Society of Cardiology (SFC), French Association of Private Cardiologists (CNCF), French Association of Hospital Cardiologists (CNCH), French Society of Thoracic and Cardiovascular Surgery (SFCTCV) and French Society of Vascular and Endovascular Surgery (SCVE)
IF 2.3
3区 医学
Q2 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
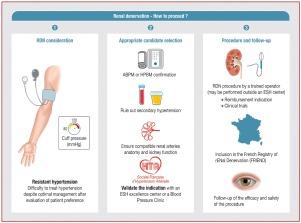
Several high-quality, randomized, sham-controlled trials have provided evidence supporting the efficacy and safety of radiofrequency, ultrasound and alcohol catheter-based renal denervation (RDN) for reducing blood pressure (BP). A French clinical consensus document has therefore been developed to propose guidance for the appropriate use of RDN in the management of hypertension along with a dedicated care pathway and management strategy. The French experts group concluded that RDN can serve as an adjunct therapy for patients with confirmed uncontrolled, resistant essential hypertension despite treatment with ≥ 3 antihypertensive drugs, including a long-acting calcium channel blocker, a renin-angiotensin system blocker and a thiazide/thiazide-like diuretic at maximally tolerated doses. Patients should have (1) an estimated glomerular filtration rate of ≥ 40 mL/min/1.73 m2; (2) an eligible renal artery anatomy on pre-RDN scans and (3) exclusion of secondary forms of hypertension. Additional indications might be considered for patients with difficult-to-control hypertension. Any indication of RDN should be validated by multidisciplinary hypertension teams consisting of both hypertension specialists and endovascular interventionalists in European Society of Hypertension (ESH) Excellence Centres or ESH-BP clinics. Patients should be informed about the benefit/risk ratio of RDN. Expertise in renal artery interventions and training in RDN techniques are needed for endovascular interventionalists conducting RDN procedures while centres offering RDN should have the necessary resources to manage potential complications effectively. Lastly, all patients undergoing RDN should have their data collected in a nationwide French registry to facilitate monitoring and evaluation of RDN outcomes, contributing to ongoing research and quality improvement efforts.
治疗动脉高血压的导管肾神经支配:代表法国高血压学会 (SFHTA)、法国放射学会 (SFR)、法国介入心脏病学会 (GACI)、法国心脏病学会 (SFC)、法国私人心脏病专家协会 (CNCF)、法国医院心脏病专家协会 (CNCH)、法国胸腔和心血管外科学会 (SFCTCV) 以及法国血管和血管内外科学会 (SCVE) 的专家共识声明。
几项高质量的随机假对照试验为射频、超声波和酒精导管肾脏去神经(RDN)降低血压(BP)的有效性和安全性提供了证据支持。因此,法国制定了一份临床共识文件,提出了在高血压治疗中适当使用 RDN 的指导意见,以及专门的护理路径和管理策略。法国专家组得出结论认为,RDN 可作为一种辅助疗法,用于在使用≥3 种降压药物(包括长效钙通道阻滞剂、肾素-血管紧张素系统阻滞剂和噻嗪类/噻嗪类利尿剂)治疗后仍无法控制的、耐药的原发性高血压患者。患者应具备以下条件:(1)估计肾小球滤过率≥40mL/min/1.73m2;(2)RDN 前扫描的肾动脉解剖符合要求;(3)排除继发性高血压。对于难以控制的高血压患者,可考虑其他适应症。任何 RDN 适应症都应由欧洲高血压学会(ESH)卓越中心或 ESH-BP 诊所的高血压专家和血管内介入专家组成的多学科高血压团队进行验证。应告知患者 RDN 的获益/风险比。进行 RDN 手术的血管内介入医师需要具备肾动脉介入方面的专业知识,并接受过 RDN 技术培训,而提供 RDN 的中心应具备必要的资源,以有效控制潜在并发症。最后,所有接受 RDN 治疗的患者的数据都应被收集到法国全国范围的登记册中,以便对 RDN 的治疗效果进行监测和评估,从而为正在进行的研究和质量改进工作做出贡献。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of Cardiovascular Diseases
医学-心血管系统
CiteScore
4.40
自引率
6.70%
发文量
87
审稿时长
34 days
期刊介绍:
The Journal publishes original peer-reviewed clinical and research articles, epidemiological studies, new methodological clinical approaches, review articles and editorials. Topics covered include coronary artery and valve diseases, interventional and pediatric cardiology, cardiovascular surgery, cardiomyopathy and heart failure, arrhythmias and stimulation, cardiovascular imaging, vascular medicine and hypertension, epidemiology and risk factors, and large multicenter studies. Archives of Cardiovascular Diseases also publishes abstracts of papers presented at the annual sessions of the Journées Européennes de la Société Française de Cardiologie and the guidelines edited by the French Society of Cardiology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


